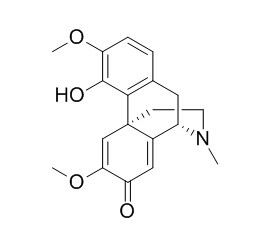
Sinoacutine
Sinoacutine has protective effects against hydrogen peroxide-induced cell injury.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Anim Cells Syst (Seoul).2024, 28(1):381-391.
Nutrients.2024, 16(15):2518.
Herbal Formula Science2024, 32(2):155-179.
Food Chem.2023, 404(Pt A):134517.
Asian J Beauty Cosmetol2020, 18(3): 265-272.
Nat Commun.2023, 14(1):5075.
Food Chem.2017, 221:1135-1144
Industrial Crops and Products2024, 219:119123
LWT2020, 110397
Data Science for Genomics2023, 107-128.
Related and Featured Products
J Biol Chem. 2009 Sep 25;284(39):26758-67.
Removal of substrate inhibition and increase in maximal velocity in the short chain dehydrogenase/reductase salutaridine reductase involved in morphine biosynthesis.[Pubmed:
19648114]
METHODS AND RESULTS:
Sinoacutine reductase (SalR, EC 1.1.1.248) catalyzes the stereospecific reduction of Sinoacutine to 7(S)-salutaridinol in the biosynthesis of morphine. In addition, a specific role in Sinoacutine binding by either hydrogen bond formation or hydrophobic interactions was assigned to each amino acid. Substrate docking also revealed an alternative mode for Sinoacutine binding, which could explain the strong substrate inhibition of Sinoacutine reductase. An alternate arrangement of Sinoacutine in the enzyme was corroborated by the effect of various amino acid substitutions on substrate inhibition. In most cases, the complete removal of substrate inhibition was accompanied by a substantial loss in enzyme activity. However, some mutations greatly reduced substrate inhibition while maintaining or even increasing the maximal velocity.
CONCLUSIONS:
Based on these results, a double mutant of Sinoacutine reductase was created that exhibited the complete absence of substrate inhibition and higher activity compared with wild-type Sinoacutine reductase.
J Nat Prod. 2005 Jul;68(7):1128-30.
Morphinane alkaloids with cell protective effects from Sinomenium acutum.[Pubmed:
16038566 ]
METHODS AND RESULTS:
One new morphinane alkaloid, sinomenine N-oxide (1), and one new natural occurring morphinane alkaloid, N-demethylsinomenine (2), together with six known alkaloids, 7,8-didehydro-4-hydroxy-3,7-dimethoxymorphinan-6-ol (3), sinomenine (4), Sinoacutine (5), N-norSinoacutine, acutumine, and acutumidine, were isolated from the stems of Sinomenium acutum. Their structures were elucidated on the basis of spectroscopic analysis and chemical methods.
CONCLUSIONS:
Compounds 2, 3, and 5 have protective effects against hydrogen peroxide-induced cell injury.
J Biol Chem. 2009 Sep 4;284(36):24432-42.
CYP719B1 is salutaridine synthase, the C-C phenol-coupling enzyme of morphine biosynthesis in opium poppy.[Pubmed:
19567876]
The C-C phenol-coupling reaction along the biosynthetic pathway to morphine in opium poppy is catalyzed by the cytochrome P450-dependent oxygenase Sinoacutine synthase.
METHODS AND RESULTS:
We report herein on the identification of Sinoacutine synthase as a member of the CYP719 family of cytochromes P450 during a screen of recombinant cytochromes P450 of opium poppy functionally expressed in Spodoptera frugiperda Sf9 cells. Recombinant CYP719B1 is a highly stereo- and regioselective enzyme; of forty-one compounds tested as potential substrates, only (R)-reticuline and (R)-norreticuline resulted in formation of a product (Sinoacutine and norsalutaridine, respectively).
J Biol Chem. 2011 Feb 25;286(8):6532-41.
Atomic structure of Sinoacutine reductase from the opium poppy (Papaver somniferum).[Pubmed:
21169353]
The opium poppy (Papaver somniferum L.) is one of the oldest known medicinal plants. In the biosynthetic pathway for morphine and codeine, salutaridine is reduced to salutaridinol by salutaridine reductase (SalR; EC 1.1.1.248) using NADPH as coenzyme.
METHODS AND RESULTS:
Here, we report the atomic structure of SalR to a resolution of ∼1.9 Å in the presence of NADPH. The core structure is highly homologous to other members of the short chain dehydrogenase/reductase family. The major difference is that the nicotinamide moiety and the substrate-binding pocket are covered by a loop (residues 265-279), on top of which lies a large "flap"-like domain (residues 105-140). This configuration appears to be a combination of the two common structural themes found in other members of the short chain dehydrogenase/reductase family. Previous modeling studies suggested that substrate inhibition is due to mutually exclusive productive and nonproductive modes of substrate binding in the active site. This model was tested via site-directed mutagenesis, and a number of these mutations abrogated substrate inhibition. However, the atomic structure of SalR shows that these mutated residues are instead distributed over a wide area of the enzyme, and many are not in the active site.
CONCLUSIONS:
To explain how residues distal to the active site might affect catalysis, a model is presented whereby SalR may undergo significant conformational changes during catalytic turnover.