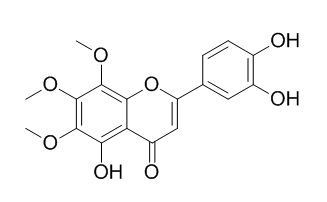
Sideritoflavone
Sideritoflavone has anti-inflammatory effect, it is a selective inhibitor of lipoxygenase activity in vitro.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2021, 22(17):9400.
Anticancer Res.2022, 42(9):4403-4410.
LWT-Food Science and Technology2017, 75:488-496
Applied Food Research2024, 100662.
Molecules.2022, 27(19):6681.
SBRAS2016, 12
Oxid Med Cell Longev2020, 12
Agronomy 2021, 11(3),502.
Int J Mol Sci.2017, 19(1)
RSC Advances2017, 86
Related and Featured Products
Agents Actions. 1991 Mar;32(3-4):283-8.
Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids.[Pubmed:
1650522]
A group of flavonoids isolated from medicinal plants and which are selective inhibitors of lipoxygenase activity in vitro: Sideritoflavone, cirsiliol, hypolaetin-8-O-beta-D-glucoside, hypolaetin, oroxindin, quercetagetin-7-O-beta-D-glucoside, gossypin, hibifolin and gossypetin, besides leucocyanidol, have been studied for their effects on acute responses induced by carrageenin in mice.
METHODS AND RESULTS:
The oral administration of flavonoids to mice inhibited dose-dependently the development of paw oedema at 1, 3 and 5 h after carrageenin injection. A similar administration of flavonoids induced a dose-dependent inhibition of leukocyte accumulation in inflammatory exudates following intraperitoneal injection of carrageenin into mice. Some of the flavonoids exhibited a potency against leukocyte infiltration similar to that seen for inhibition of carrageenin oedema at 3 h of induction. In agreement with data reported in rats, indomethacin was much more effective on inhibition of prostaglandin E2 (PGE2) formation than on leukocyte infiltration in mice. The selectivity of flavonoids towards lipoxygenase is not retained in vivo since they behave as dual inhibitors of PGE2 and leukotriene B4 (LTB4) formation in peritoneal exudates.
CONCLUSIONS:
Our data support the inhibition of arachidonic acid metabolism as one of the mechanisms by which flavonoids exert their anti-inflammatory effects.
J Nat Prod. 2015 Jan 23;78(1):69-76.
Methoxyflavones from Stachys glutinosa with binding affinity to opioid receptors: in silico, in vitro, and in vivo studies.[Pubmed:
25562563 ]
Fractionation of the bioactive dichloromethane extract from the aerial parts of Stachys glutinosa led to the isolation of four flavones, xanthomicrol (1), Sideritoflavone (2), 8-methoxycirsilineol (3), and eupatilin (4), along with two neo-clerodane diterpenes, roseostachenone (8) and a new compound, 3α,4α-epoxyroseostachenol (7). In order to study structure-activity relationships, two methoxyflavones [5-demethyltangeretin (5) and tangeretin (6)] were synthesized by the methoxylation of xanthomicrol.
METHODS AND RESULTS:
The isolated compounds (1-4, 7, and 8) as well as the xanthomicrol semisynthetic derivatives (5 and 6) were evaluated for their binding affinity to the μ and δ opioid receptors. Xanthomicrol was the most potent binder to both μ and δ receptors, with a Ki value of 0.83 and 3.6 μM, respectively. Xanthomicrol administered intraperitoneally in mice at a dose of 80 mg/kg significantly reduced morphine-induced antinociception in the tail flick test. Our results suggested that xanthomicrol is a μ opioid receptor antagonist. Docking experiments were carried out to acquire a deeper understanding about important structural aspects of binding of xanthomicrol.
CONCLUSIONS:
In summary, these data suggest that xanthomicrol is a valuable structure for further development into a potential μ opioid receptor antagonist.