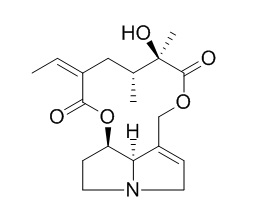
Senecionine
Senecionine (SEN) is a representative of the hepatotoxic pyrrolizidine alkaloids.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Arch Biochem Biophys.2018, 644:93-99
Phytochemistry2018, 15:83-92
Int J Mol Sci.2018, 19(9):E2528
Sci Rep.2017, 7:46299
Mol Neurobiol.2023, 60(12):7196-7207.
Cell Physiol Biochem.2017, 44(4):1381-1395
Metabolites.2020, 11(1):E11.
Oxid Med Cell Longev.2022, 2022:9139338.
PLoS One.2022, 17(4):e0267007.
Food Research International2023, 113792.
Related and Featured Products
Biochem Pharmacol. 1989 Feb 1;38(3):391-7.
Effects of the pyrrolizidine alkaloid senecionine and the alkenals trans-4-OH-hexenal and trans-2-hexenal on intracellular calcium compartmentation in isolated hepatocytes.[Pubmed:
2492804]
The pyrrolizidine alkaloid Senecionine has been shown to produce an increase in cytosolic free Ca2+ concentration in isolated hepatocytes that correlated with an increase in cellular toxicity.
METHODS AND RESULTS:
The cytotoxicity was greater in the absence of extracellular Ca2+ than in its presence, suggesting that alterations in intracellular Ca2+ distribution, and not an influx of extracellular Ca2+, were responsible for the Senecionine-induced hepatotoxicity. The effect of Senecionine, as well as the effects of trans-4-OH-2-hexenal (t-4HH), a microsomal metabolite of Senecionine, and a related alkenal, trans-2-hexenal, on the sequestration of Ca2+ in mitochondrial and extramitochondrial compartments were examined in isolated hepatocytes. Each of the test compounds elicited a decrease in the available extramitochondrial Ca2+ stores that was inhibited by pretreatment with the thiol group reducing agent, dithiothreitol. Senecionine and t-4HH decreased the level of Ca2+ sequestered in the mitochondrial compartment of hepatocytes. The presence of a pyridine nucleotide reducing agent, beta-hydroxybutyrate, inhibited this reduction.
CONCLUSIONS:
These results suggest that both Senecionine and t-4HH inhibit the sequestration of Ca2+ in extramitochondrial and mitochondrial compartments possibly by inactivating free sulfhydryl groups and oxidizing pyridine nucleotides respectively.
Chem Res Toxicol. 2014 May 19;27(5):775-86.
Metabolomic and genomic evidence for compromised bile acid homeostasis by senecionine, a hepatotoxic pyrrolizidine alkaloid.[Pubmed:
24641316]
METHODS AND RESULTS:
Senecionine hepatotoxicity on rats was determined via a combination of metabolomic and genomic analyses. From the global analysis generated from two omics data, the compromised bile acid homeostasis in vivo was innovatively demonstrated and confirmed. Serum profiling of bile acids was altered with significantly elevated conjugated bile acids after Senecionine exposure, which was in accordance with toxicity. Similarly, the hepatic mRNA levels of several key genes associated with bile acid metabolism were significantly changed.
CONCLUSIONS:
In conclusion, a cross-omics study provides a comprehensive analysis method for studying the toxicity caused by Senecionine, which is a hepatotoxic PA. Moreover, the change in bile acid metabolism and the respective transporters may provide a new PA toxicity mechanism.
Carcinogenesis. 1991 Mar;12(3):515-9.
Role of cytochrome P450IIIA4 in the metabolism of the pyrrolizidine alkaloid senecionine in human liver.[Pubmed:
2009596]
Studies were carried out to investigate the metabolism of Senecionine by human liver microsomes and the role of human cytochrome P450IIIA4 in this process.
METHODS AND RESULTS:
Human liver microsomes metabolized Senecionine to two major products, (+/-)-6,7-dihydro-7-hydroxy-1-hydroxymethyl-5H-pyrrolizine (DHP) and Senecionine N-oxide. The rates of product formation (DHP and Senecionine N-oxide) varied widely with the microsomal samples tested. There was a 30-fold difference in DHP formation and a 25-fold difference in N-oxidation between the poorest metabolizer and the highest metabolizer of Senecionine. The conversion of Senecionine to DHP and Senecionine N-oxide in human liver microsomes was markedly inhibited by the mechanism-based inactivators of P450IIIA4, gestodene and triacetyloleandomycin. Anti-P450IIIA4 IgG, at a concentration of 1 mg/nmol of P450, was found to inhibit completely the formation of DHP and Senecionine N-oxide in human liver microsomes (HL101) having low activity toward Senecionine. At 5 mg IgG/nmol P450, anti-P450IIIA4 inhibited 90 and 84% respectively of the formation of DHP and Senecionine N-oxide in liver microsomes (HL110) with the highest activity toward Senecionine. The formation of DHP or Senecionine N-oxide was highly correlated with the amount of P450IIIA4 measured in the microsomes using polyclonal anti-P450IIIA4 IgG. The rate of DHP production also had a strong correlation with the rate of Senecionine N-oxide formation (r = 0.999) and with the rate of nifedipine oxidation (r = 0.998).
CONCLUSIONS:
Our present studies provide evidence that P450IIIA4 is the major enzyme catalyzing the bioactivation (DHP formation) and detoxication (Senecionine N-oxide formation) of Senecionine in human liver.
Drug Metab Dispos. 2010 Apr;38(4):626-34.
Identification of the UDP-glucuronosyltransferase isozyme involved in senecionine glucuronidation in human liver microsomes.[Pubmed:
20056725]
Senecionine (SEN) is a representative of the hepatotoxic pyrrolizidine alkaloids. Although phase I metabolism for cytochrome P450-mediated metabolic activation of Senecionine was investigated extensively, phase II metabolism for glucuronidation of this compound has not been investigated until now.
METHODS AND RESULTS:
In our present study, one unique glucuronidation product of Senecionine in human liver microsomes (HLMs) was identified as Senecionine N-glucuronide using an authentically synthesized product for which the structure was identified via (1)H and (13)C NMR analysis. Subsequently, kinetics indicated that Senecionine N-glucuronidation followed the typical Michaelis-Menten model and only one major isozyme participated in it. Finally, this isozyme was demonstrated to be UDP-glucuronosyltransferase (UGT) 1A4, with the direct evidence that recombinant UGT1A4 exhibited predominant and exclusive activity on Senecionine N-glucuronidation. This result was confirmed by other experiments including chemical inhibition by selective inhibitors and a correlation study between activities of Senecionine N-glucuronidation and various UGT isozymes.
CONCLUSIONS:
The exclusive role of UGT1A4 on Senecionine N-glucuronidation was strengthened additionally by its inhibitory kinetic study in which the selective inhibitor of UGT1A4 showed a similar inhibition pattern and K(i) values in both HLM and recombinant UGT1A4 systems.
Because UGT2B10 activity failed to correlate with Senecionine N-glucuronidation in HLMs from 10 individuals, it was impossible for UGT2B10 to play an important role in this metabolism.