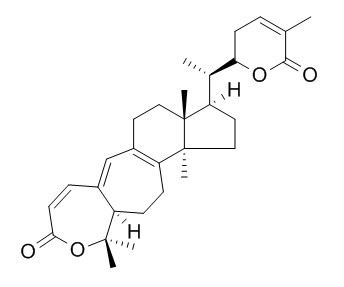
Schisanlactone A
Schisanlactone A is a dimerization inhibitor, with an IC50 value of 5.0 microg/mL; it also shows appreciable inhibitory activity against HIV-1 protease with the IC50 value of 20 microM. Schisanlactone A shows cytotoxicity against KB (a human epidermal carcinoma) cells with the IC50 value of 63.3 uM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules. 2013, 18(7):7376-88
Integrative Medicine Research2024, 13(1):101025.
J Sep Sci.2018, 41(9):1938-1946
ScienceAsia2024, 50,2024073:1-9
Neurochem Int.2023, 167:105537.
Appl. Sci. 2021, 11(23),11099.
Ann Transl Med.2019, 7(23):731
Korean J Dent Mater.2018, 45(2):139-146
Biomimetics (Basel).2022, 7(4):154.
Environ Toxicol.2020, doi: 10.1002
Related and Featured Products
J Nat Prod. 2009 Nov;72(11):2019-23.
Inhibition of the dimerization and active site of HIV-1 protease by secondary metabolites from the Vietnamese mushroom Ganoderma colossum.[Pubmed:
19813754 ]
METHODS AND RESULTS:
A new farnesyl hydroquinone, ganomycin I (1), was isolated along with ganomycin B (2) from the chloroform extract of the fruiting bodies of the Vietnamese mushroom Ganoderma colossum. These compounds inhibited HIV-1 protease with IC50 values of 7.5 and 1.0 microg/mL, respectively.
CONCLUSIONS:
Kinetic studies using Zhang-Poorman and Lineweaver plots revealed that compound 2 competitively inhibited the active site of the enzyme, whereas the tetracyclic triterpene Schisanlactone A, previously isolated from the same fungus, was a dimerization inhibitor, with an IC50 value of 5.0 microg/mL. The previous findings were also confirmed by the virtual docking of both compounds with HIV-1 protease crystal structure.
Fitoterapia. 2013 Dec;91:125-7.
Cattienoids A-C, three novel steroids from the mushroom Tomophagus cattienensis.[Pubmed:
24001711 ]
METHODS AND RESULTS:
Three novel steroids (1-3), named cattienoids A-C together with Schisanlactone A (4), were isolated from fruiting bodies of Tomophagus cattienensis (Ganodermataceae)-a new mushroom recently collected from Cattien National Park, in Viet Nam. They possess an unusual seven membered lactone ring, derived from lanostane-type triterpenoids. Their structures were determined by spectroscopic methods. In addition, compounds 2 and 4 showed cytotoxicity against KB (a human epidermal carcinoma) cells with their IC50 values of 91.2 and 63.3 μM, respectively.
CONCLUSIONS:
These compounds have neither antimicrobial activity nor inhibition of the tyrosinase.
Chem Pharm Bull (Tokyo). 2006 Jan;54(1):129-32.
Three new lignans, longipedunins A-C, from Kadsura longipedunculata and their inhibitory activity against HIV-1 protease.[Pubmed:
16394567]
METHODS AND RESULTS:
Three new lignans, longipedunins A (1), B (2) and C (3), together with three known compounds, benzoyl-binankadsurin A (4), acetyl-binankadsurin A (5) and Schisanlactone A (6), were isolated from Kadsura longipedunculata. Their structures and stereochemistry were determined by spectral and single-crystal X-ray analyses.
CONCLUSIONS:
Compounds 1 and 6 showed appreciable inhibitory activity against HIV-1 protease with IC50 values of 50 and 20 microM, respectively.