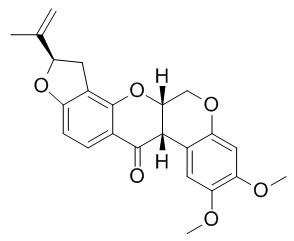
Rotenone
Rotenone is a mitochondrial complex I inhibitor that produces an animal model of Parkinson's disease. Rotenone-induced α-synuclein aggregation is mediated by the calcium/GSK3β signaling pathway. Rotenone can increase intracellular levels of the toxic dopamine metabolite 3,4-dihydroxyphenyl-acetaldehyde (DOPAL), via decreasing DOPAL metabolism by aldehyde dehydrogenase (ALDH) and decreasing vesicular sequestration of cytoplasmic dopamine by the vesicular monoamine transporter (VMAT).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Trop J Nat Prod Res, February2023, 7(2):2371-2381
Korean J. Crop Sci.2018, 63(2):131-139
Acta Agriculturae Scandinavica2015, 381-383
J Clin Med.2019, 8(10):E1664
Korean Herb. Med. Inf.2020, 8(2):243-254.
Food Bioscience2023, 53:102687
Sci Rep.2019, 9(1):6429
Neuroscience.2024, 559:77-90.
Molecules2021, 26(1),230
Chemistry of Plant Materials.2019, 215-222
Related and Featured Products
Toxicol Lett. 2015 May 5;234(3):162-71.
GAPDH-knockdown reduce rotenone-induced H9C2 cells death via autophagy and anti-oxidative stress pathway.[Pubmed:
25725130]
GAPDH, well known for its house-keeping functions, has also been shown to be involved in cell injury, apoptosis and death under conditions of stress such as starvation, chemical injury and oxidative stress. This study examines the effect of GAPDH knockdown on cell injury in response to Rotenone.
METHODS AND RESULTS:
GAPDH was knocked down in H9C2 cardiomyoblasts using siRNA prior to exposure to Rotenone (0 nM, 20 nM, 40 nM and 80 nM). Autophagy was detected by western blot for autophagy proteins (Beclin-1, Atg5, LC-3A/B and p62) and MDC staining for acidic substances. Pro-apoptosis protein and flow cytometry were used to assess cell apoptosis and death and intracellular ATP relative concentration was measured. Oxidant stress was assessed by measuring DCFH-DA, TBARS, GSH and SOD.
In this study, GAPDH-knockdown enhanced autophagy in Rotenone-induced H9C2 cells, decreased oxidant stress and increased antioxidant pathways; and reduced cell apoptosis and death. Furthermore, GAPDH-knockdown preserved cell energy.
CONCLUSIONS:
siRNA-mediated GAPDH knockdown reduced Rotenone-induced H9C2 cell death occurring via autophagy and anti-oxidative stress pathway. This study enriches the understanding of GAPDH pathophysiology role, and provides potential new therapeutic targets for cardiac disease states characterized by oxidative stress.
Toxicology. 2015 Feb 3;328:75-81.
JNK inhibition of VMAT2 contributes to rotenone-induced oxidative stress and dopamine neuron death.[Pubmed:
25496994]
Treatment with Rotenone, both in vitro and in vivo, is widely used to model dopamine neuron death in Parkinson's disease upon exposure to environmental neurotoxicants and pesticides. Mechanisms underlying Rotenone neurotoxicity are still being defined.
METHODS AND RESULTS:
Our recent studies suggest that Rotenone-induced dopamine neuron death involves microtubule destabilization, which leads to accumulation of cytosolic dopamine and consequently reactive oxygen species (ROS). Furthermore, the c-Jun N-terminal protein kinase (JNK) is required for Rotenone-induced dopamine neuron death. Here we report that the neural specific JNK3 isoform of the JNKs, but not JNK1 or JNK2, is responsible for this neuron death in primary cultured dopamine neurons. Treatment with taxol, a microtubule stabilizing agent, attenuates Rotenone-induced phosphorylation and presumably activation of JNK. This suggests that JNK is activated by microtubule destabilization upon Rotenone exposure. Moreover, Rotenone inhibits VMAT2 activity but not VMAT2 protein levels. Significantly, treatment with SP600125, a pharmacological inhibitor of JNKs, attenuates Rotenone inhibition of VMAT2. Furthermore, decreased VMAT2 activity following in vitro incubation of recombinant JNK3 protein with purified mesencephalic synaptic vesicles suggests that JNK3 can inhibit VMAT2 activity.
CONCLUSIONS:
Together with our previous findings, these results suggest that Rotenone induces dopamine neuron death through a series of sequential events including microtubule destabilization, JNK3 activation, VMAT2 inhibition, accumulation of cytosolic dopamine, and generation of ROS. Our data identify JNK3 as a novel regulator of VMAT2 activity.
Toxicol Lett. 2015 Mar 4;233(2):163-71.
The molecular mechanism of rotenone-induced α-synuclein aggregation: emphasizing the role of the calcium/GSK3β pathway.[Pubmed:
25433145]
Environmental toxin exposure is associated with the development of Parkinson's disease (PD), and environmental factors can influence the onset of the majority of sporadic PD cases via genetically mediated pathways. Rotenone, a widespread pesticide, induces Parkinsonism and the formation of Lewy bodies in animals; however, the molecular mechanism that underlies α-synuclein aggregation remains unclear.
METHODS AND RESULTS:
Here, we assessed the aggregation of α-synuclein in PC12 cells with or without cross-linking following Rotenone exposure via a variety of methods, including western blotting, immunofluorescence and electron microscopy. We demonstrated that Rotenone increased the intracellular calcium levels and induced the aggregation and phosphorylation of α-synuclein in a calcium-dependent manner. Aggregated α-synuclein is typically degraded by autophagy, and Rotenone impaired this process. The attenuation of autophagy and α-synuclein alterations were reversed by scavenging calcium. Calcium regulates the activity of AKT-glycogen synthase kinase 3 (GSK3)β. We demonstrated that Rotenone attenuated the phosphorylation of AKT and GSK3β, and the elimination of calcium reversed these phenomena. As a GSK3β inhibitor, lithium promoted autophagy and decreased the aggregation and phosphorylation of α-synuclein. GSK3β activation through overexpression depressed autophagy and increased the total protein level and phosphorylation of α-synuclein.
CONCLUSIONS:
These results suggest that Rotenone-induced α-synuclein aggregation is mediated by the calcium/GSK3β signaling pathway.
J Neurochem. 2015 Apr;133(1):14-25.
Rotenone decreases intracellular aldehyde dehydrogenase activity: implications for the pathogenesis of Parkinson's disease.[Pubmed:
25645689]
Repeated systemic administration of the mitochondrial complex I inhibitor Rotenone produces a rodent model of Parkinson's disease (PD). Mechanisms of relatively selective Rotenone-induced damage to nigrostriatal dopaminergic neurons remain incompletely understood.
According to the 'catecholaldehyde hypothesis,' buildup of the autotoxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde (DOPAL) contributes to PD pathogenesis. Vesicular uptake blockade increases DOPAL levels, and DOPAL is detoxified mainly by aldehyde dehydrogenase (ALDH).
METHODS AND RESULTS:
We tested whether Rotenone interferes with vesicular uptake and intracellular ALDH activity. Endogenous and F-labeled catechols were measured in PC12 cells incubated with Rotenone (0-1000 nM, 180 min), without or with F-dopamine (2 μM) to track vesicular uptake and catecholamine metabolism. Rotenone dose dependently increased DOPAL, F-DOPAL, and 3,4-dihydroxyphenylethanol (DOPET) levels while decreasing dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC) levels and the ratio of dopamine to the sum of its deaminated metabolites. In test tubes, Rotenone did not affect conversion of DOPAL to DOPAC by ALDH when NAD(+) was supplied, whereas the direct-acting ALDH inhibitor benomyl markedly increased DOPAL and decreased DOPAC concentrations in the reaction mixtures. We propose that Rotenone builds up intracellular DOPAL by decreasing ALDH activity and attenuating vesicular sequestration of cytoplasmic catecholamines.
CONCLUSIONS:
The results provide a novel mechanism for selective Rotenone-induced toxicity in dopaminergic neurons. We report that Rotenone, a mitochondrial complex I inhibitor that produces an animal model of Parkinson's disease, increases intracellular levels of the toxic dopamine metabolite 3,4-dihydroxyphenyl-acetaldehyde (DOPAL), via decreased DOPAL metabolism by aldehyde dehydrogenase (ALDH) and decreased vesicular sequestration of cytoplasmic dopamine by the vesicular monoamine transporter (VMAT). The results provide a novel mechanism for Rotenone-induced toxicity in dopaminergic neurons.