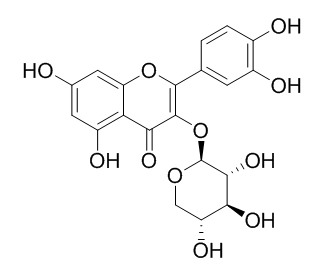
Reynoutrin
Reynoutrin has antioxidant activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Microorganisms.2021, 9(12):2514.
Kyung Hee University2024, rs-3888374
Genes Environ.2024, 46(1):13.
Plants (Basel).2023, 12(11):2107.
Proc Biol Sci.2024, 291(2015):20232578.
Pharmacognosy Journal2019, 11(2): 369-373
Br J Pharmacol.2024, 181(24):5009-5027.
Food Bioscience2024, 57:103518.
Ethnomedicinal Plants for Drug Discovery2024, 491-509
Res Pharm Sci.2023, 18(3):244-261.
Related and Featured Products
Nat Prod Res. 2012;26(3):274-7.
Antioxidant potential and radical-scavenging effects of flavonoids from the leaves of Psidium cattleianum grown in French Polynesia.[Pubmed:
22077157 ]
Psidium cattleianum J. Sabine (Myrtaceae) is a traditional medicinal plant in French Polynesia. The leaves and roots possess many medicinal properties. These effects may be correlated with the presence of antioxidant compounds.
METHODS AND RESULTS:
Seven flavonoids along with a benzoic acid were isolated from the leaves of P. cattleianum. The compounds indicated strong antioxidant and radical-scavenging activities in ALP, DPPH(·), ABTS(·-) and ORAC assays. This study demonstrates that the leaves of P. cattleianum possess main compounds with interesting antioxidant and radical-scavenging activities, as clarified by four biological assays.
CONCLUSIONS:
Our findings may justify the use of these leaves in the traditional medicine of French Polynesia. Among the total eight known compounds, Reynoutrin and luteolin were isolated for the first time from the genus Psidium.
Food Chem. 2008 Mar 15;107(2):813-819.
Cytotoxic chalcones and antioxidants from the fruits of a Syzygium samarangense (Wax Jambu).[Pubmed:
22359426 ]
METHODS AND RESULTS:
Bioassay-guided fractionation of the methanolic extracts of the pulp and seeds of the fruits of Syzygium samarangense Merr. & Perry (Blume) led to the identification of four cytotoxic compounds and eight antioxidants on the basis of HPLC-PDA analysis, MS, and various NMR spectroscopic techniques.
CONCLUSIONS:
Three C-methylated chalcones, 2',4'-dihydroxy-3',5'-dimethyl-6'-methoxychalcone (1), 2',4'-dihydroxy-3'-methyl-6'-methoxychalcone (stercurensin, 2), and 2',4'-dihydroxy-6'-methoxychalcone (cardamonin, 3), were isolated and displayed cytotoxic activity (IC(50) = 10, 35, and 35 μM, respectively) against the SW-480 human colon cancer cell line. Also a number of known antioxidants were obtained including six quercetin glycosides: Reynoutrin (4), hyperin (5), myricitrin (6), quercitrin (7), quercetin (9), and guaijaverin (10), one flavanone: (S)-pinocembrin (8), and two phenolic acids: gallic acid (11) and ellagic acid (12).
J Nat Prod. 1999 May;62(5):705-9.
Geranins A and B, new antiprotozoal A-type proanthocyanidins from Geranium niveum.[Pubmed:
10346950 ]
METHODS AND RESULTS:
Bioassay-guided fractionation of the antiprotozoal extract of Geranium niveum led to the isolation of two new A-type proanthocyanidins, epi-afzelechin-(4beta-->8, 2beta-->O-->7)-afzelechin (1) and epi-catechin-(4beta-->8, 2beta-->O-->7)-afzelechin (2). Compounds 1 and 2 were given the trivial names of geranins A and B, respectively. In addition, five known compounds, mahuannin B (3), Reynoutrin (4), hyperin (5), methyl gallate (6), and 3-beta-caffeoyl-12-oleanen-28-oic acid (7), were obtained.
The structures of the new proanthocyanidins were elucidated by spectroscopic and chemical methods as well as CD measurements.
CONCLUSIONS:
Compounds 1, 2, and 4-7 were tested against axenically grown trophozoites of Giardia lamblia and Entamoeba histolytica.