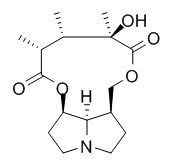
Retusine
Retusine has edema inhibition capacity, could be related to a reduction of the prostaglandin production. Retusine shows activity against the Gram-positive bacteria C. diphtheria and S. aureus.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2019, 24(7):E1290
J Nat Prod.2018, 81(4):966-975
J Chromatogr B Analyt Technol Biomed Life Sci. 2017, 1064:115-123
Appl. Sci.2021, 11(24),12080
Semyung University2017, 149407
Plants (Basel).2024, 13(6):868.
Food Chem Toxicol.2024, 186:114589.
Separations2021, 8(1), 1.
Data Science for Genomics2023, 107-128.
Viruses2023, 15(6), 1377
Related and Featured Products
Z Naturforsch C. 2004 Mar-Apr;59(3-4):237-43.
Anti-inflammatory constituents of Mortonia greggii Gray.[Pubmed:
15241934 ]
A new phytochemical study of Mortonia greggii (Celastraceae) afforded four friedelan derivatives (1-4), three lupanes (5-7), Retusine (8), two esterified polyhydroxyagarofurans (9-10), mortonin C (11) and photomortonin C (12).
METHODS AND RESULTS:
The anti-inflammatory activity on carrageenan and 12-O-tetradecanoylphorbol-13-acetate induced models of inflammation, as well as the ability to inhibit the nitric oxide (NO) produced by lipopolysaccharide-stimulated mouse peritoneal macrophages were evaluated for the main metabolites. Our results showed that the friedelan dehydrocanophyllic acid methyl ester (1) exhibits an anti-inflammatory effect which could be related to an inhibition of prostaglandin and NO production. The activity of lupeol (5), 29-hydroxylupeol (6) and 29-hydroxylupenone (7) might be involved with the prostanoid synthesis. The presence of the hydroxy groups in 6 appears to be important for activity.
CONCLUSIONS:
The edema inhibition capacity of Retusine (8) could be related to a reduction of the prostaglandin production. The agarofuran derivative 10 is an NO inhibitor whose activity is probably not involved in the synthesis of prostaglandins.
Ann Pharm Fr. 1997;55(6):262-8
Isolation, identification and antimicrobial activity of ombuoside from Stevia triflora.[Pubmed:
9453171]
From aerial parts of Stevia triflora DC the flavonol glycoside ombuoside (7,4'-di-O-methylquercetin-3-O-beta-rutinoside) has been isolated and identified on the basis of spectral data.
METHODS AND RESULTS:
Ombuoside and the synthetic derivatives octa-acetylombuoside, ombuine and Retusine were tested for antimicrobial activity against several strains of Gram-positive and Gram-negative bacteria and the yeast Candida albicans, using the agar diffusion method. The flavonol glycoside ombuoside and the respective aglycone ombuine, both exhibited moderated activity against Corynebacterium diphtheria, Staphylococcus aureus, Escherichia coli and Candida albicans. To a lesser degree, octaacetylombuoside and Retusine showed activity against the Gram-positive bacteria C. diphtheria and S. aureus, but proved to be inactive against Gram-negative bacteria and Candida albicans.
CONCLUSIONS:
These results indicate that the presence of free hydroxyl groups, either alcoholic or phenolic, is an important chemical feature for the expression of flavonol antimicrobial activity. It is worth noting that this is the first study reported on the antibacterial and antifungal activity of these substances.