Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
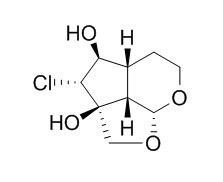
Chem Pharm Bull (Tokyo). 1994 Aug;42(8):1669-71.
Studies on the constituents of Ailanthus integrifolia.[Pubmed:
7954920]
METHODS AND RESULTS:
A new phenolic glycoside, 3,4,5-trimethoxyphenol-1-(6-xylopyranosyl)glucopyranoside, was isolated together with twenty known compounds identified as koaburaside, 3,4,5-trimethoxyphenol, 5,7-dihydroxychromone-7-neohesperidoside, naringin, neoeriocitrin, p-coumaric acid, vanillin, vanillic acid, coniferyl aldehyde, ferulic acid, trans-triacontyl-4-hydroxy-3-methoxycinnamate, p-methoxycinnamic acid, 2,6-dimethoxybenzoquinone, 2-(1-hydroxyethyl)naphtho[2,3-b]furan-4,9-dione, 2-acetylnaphtho[2,3-b]furan-4,9-dione, 2-(1-hydroxyethyl)-6-methoxynaphtho[2,3-b]furan-4,9-dione, 2-acetyl-6-methoxynaphtho[2,3-b]furan-4,9-dione, specioside, jioglutin C and Rehmaglutin D from the bark of Ailanthus integrifolia Lamk (Simaroubaceae).
Phytochemistry. 2002 Mar;59(5):537-42.
Non-glycosidic iridoids from Cymbaria mongolica.[Pubmed:
11853749]
METHODS AND RESULTS:
Six non-glycosidic iridoids, i.e. (1R,4S,4aS,7S,7aS)-7-hydroxyl-4-hydroxy- methyl-7-methyl-1-methoxyl-1,4,4a,7a-tetrahydrocyclopenta[e]pyran-3-one (1), (1S,4R,4aS,7S,7aS)-7-hydroxyl-4-hydroxymethyl-7-methyl-1-methoxyl-1,4,4a,7a-tetrahydrocyclopenta[e]pyran-3-one (2), (1R,4R,4aS,7S,7aS)-7-hydroxyl-4-hydroxy-methyl-7-methyl-1-methoxyl-1,4,4a,7a-tetrahydrocyclopenta[e]pyran-3-one (3), (1R, 4R, 4aS, 7aS)-4,7-dihydroxymethyl-1-methoxyl-1,4,4a,7a-tetrahydrocyclopenta-6-ene[e]pyran-3-one (4), (1R, 4R, 4aS, 7aS)-4,7-dihydroxymethyl-1-hydroxyl-1,4,4a, 7a-tetrahydrocyclopenta-6-ene[e]pyran-3-one (5), (1R, 4S, 4aS, 7aS)-4,7-dihydroxy-methyl-1-methoxyl-1,4,4a,7a-tetrahydrocyclopenta-6-ene[e]pyran-3-one (6), as well as five known non-glycosidic iridoids mussaenin A (7), gardendiol (8), isoboonein (9), 4-epi-alyxialactone (10) and Rehmaglutin D (11) have been isolated from the Chinese medicinal plant Cymbaria mongolica.