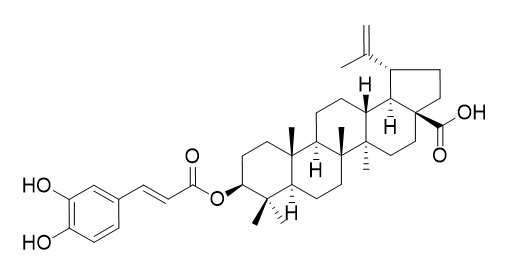
Pyracrenic acid
Pyracrenic acid is a potent elastase inhibitor with an IC50 value of 1.5 mg/mL. Pyracrenic acid shows DPPH radical scavenging activity. It also shows significant cytotoxic activities against human cancer cells COLO 205 and AGS.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Immunol.2023, ji2200727.
Pharmacogn Mag.2015, 11(43):562-6
Saudi Pharm J.2019, 27(1):145-153
Heliyon2022, 8(2):e08866.
Ann Transl Med.2019, 7(23):731
Lab Chip.2018, 18(6):971-978
Mol Biol Rep.2024, 51(1):117.
Heliyon.2023, e12684.
Sci Adv.2018, 4(10)
Cells.2023, 12(1):168.
Related and Featured Products
Chem Biodivers. 2008 Apr;5(4):565-74.
Cytotoxic triterpenoids from the root bark of Helicteres angustifolia.[Pubmed:
18421748 ]
METHODS AND RESULTS:
Three new triterpenoids, 3beta-acetoxy-27-[(E)-cinnamoyloxy]lup-20(29)-en-28-oic acid methyl ester (1), 3beta-acetoxy-27-[(4-hydroxybenzoyl)oxy]lup-20(29)-en-28-oic acid (2), and 3beta-acetoxy-27-[(4-hydroxybenzoyl)oxy]olean-12-en-28-oic acid methyl ester (3), together with nine known triterpenoids, 4-12, were isolated from the root bark of Helicteres angustifolia. The structures of these compounds were established on the basis of spectroscopic methods including 2D-NMR experiments. All twelve compounds were tested for their cytotoxic activities against human colorectal cancer (COLO 205), human hepatoma (Hep G2), and human gastric cancer (AGS) cell lines in vitro.
CONCLUSIONS:
Among them, compounds 2, 3, 3beta-O-[(E)-coumaroyl]betulinic acid (6), and Pyracrenic acid (7) showed significant cytotoxic activities against human cancer cells COLO 205 and AGS.
Journal of Medicinal Plant Research, 2009, 3(11):914-920.
Compounds with elastase inhibition and free radical scavenging activities from Callistemon lanceolatus.[Reference:
WebLink]
METHODS AND RESULTS:
Bioassay-guided investigation of the stems of Callistemon lanceolatus led to the isolation of five compounds such as betulinic acid (1), Pyracrenic acid (2), arjunolic acid (3), catechin (4), and piceatannol (5). Their structures were elucidated on the basis of spectral studies as well as by comparison with literature data. Elastase inhibition and DPPH radical scavenging activities wereinvestigated for the isolated compounds.
CONCLUSIONS:
Among these, Pyracrenic acid (2) was identified as a potent elastase inhibitor with an IC50 value of 1.5 mg/mL, more active than the control compound, oleanolic acid (IC50 = 3.0 mg/mL). With regard to antioxidative studies, strong to moderate DPPH radical scavenging activities were observed with Pyracrenic acid (2), catechin (4) and piceatanol (5).
Zhong Yao Cai. 2012 Jun;35(6):904-8.
Chemical constituents from the aerial roots of Ficus microcarpa.[Pubmed:
23236824]
To study the chemical constituents of the aerial roots of Ficus microcarpa.
METHODS AND RESULTS:
The compounds were isolated and purified by column chromatographic methods on silica gel and Sephadex LH-20. Their structures were elucidated by physicochemical properties and spectral data. RESULTS: 12 compounds were isolated from the 95% ethanol extract. Their structures were idenified as 3beta-hydroxy-11-oxours-12-ene (1), 3beta-acetoxy-11-oxours-12-ene (2), oleanic acid (3), 3beta-hydroxy-oleana-11,13 (18)-dien-28-oic acid (4), betulinic acid (5), Pyracrenic acid (6), platanic acid (7), isowigtheone (8), myrsininone A (9), derrone (10), alpinumisoflavone (11) and caffeic acid methyl ester (12), respectively.
CONCLUSIONS:
Compounds 4, 6, 12 are obtained from this genus for the first time, compounds 1, 7 - 11 are isolated from this plant for the first time.
Isonemerosin
Catalog No: CFN95008
CAS No: 181524-79-8
Price: $268/20mg
Poricoic acid G
Catalog No: CFN95056
CAS No: 415724-84-4
Price: $318/5mg
Daidzein-4'-glucoside
Catalog No: CFN95142
CAS No: 58970-69-7
Price: $318/5mg
(3S,5S,E)-1,7-Diphenylhept-1-ene-3,5-diol
Catalog No: CFN95195
CAS No: 87095-75-8
Price: $318/5mg
[(1(10)E,2R,4R)]-2-Methoxy-8,12-epoxygemacra-1(10),7,11-trien-6-one
Catalog No: CFN95198
CAS No: 75412-95-2
Price: $318/10mg
Saucerneol
Catalog No: CFN95267
CAS No: 88497-86-3
Price: $318/5mg
4,7-Didehydroneophysalin B
Catalog No: CFN95317
CAS No: 134461-76-0
Price: $338/5mg
Oxytroflavoside E
Catalog No: CFN95464
CAS No: 1391144-84-5
Price: $318/5mg
Herbacetin 3-sophoroside-8-glucoside
Catalog No: CFN95529
CAS No: 77298-68-1
Price: $318/5mg
3',3'''-Binaringenin
Catalog No: CFN95558
CAS No: 145399-99-1
Price: $413/5mg