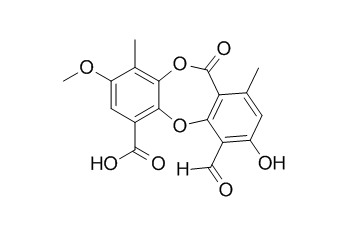
Psoromic acid
Psoromic acid is a selective and covalent rab-prenylation inhibitor targeting autoinhibited RabGGTase, it shows antibacterial activities against Streptococcus gordonii and Porphyromonas gingivalis, and it is an effective and safe natural drug plausible for use in controlling tuberculosis infections. Psoromic acid shows antioxidative and cardiovascular-protective activity, it also displays significant apoptotic activities.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Anat Rec2018, 24264
Microchemical Journal2024, 200:110475
Curr Res Food Sci.2024, 9:100827.
BMC Complement Altern Med.2014, 14:242
Cell Physiol Biochem.2019, 52(6):1255-1266
JEJU National University2022, 10478.
Antioxidants (Basel).2023, 13(1):12.
Food Funct.2023, 14(9):4354-4367.
J Korean Soc Food Sci Nutr2023, 52(7): 750-757
Pathogens.2018, 7(3):E62
Related and Featured Products
Pharmaceutical Biology, 2012, 50(8):968-979.
Antioxidative and cardiovascular-protective activities of metabolite usnic acid and psoromic acid produced by lichen species Usnea complanata under submerged fermentation.[Reference:
WebLink]
Lichens have been used for various purposes such as dyes, perfumes and remedies in folk medicine indicating the pharmaceutical potential of lichens. Lichen growth in nature is very slow. To overcome this major drawback, we standardized the culture media to culture the lichen Usnea complanata (Müll.Arg.) Motyka (Parmeliaceae) for (1) in vitro synthesis of natural lichen substances, and (2) determination of antioxidative and cardiovascular-protective activity of usnic acid and Psoromic acid.
METHODS AND RESULTS:
Lichen U. complanata has been cultured in fermentor under submerged condition. Antioxidative and cardiovascular-protective activity of the extract and the purified lichen substances usnic and Psoromic acid have been determined. Except methanol, all other extracts exhibited antioxidative action in terms of free radical scavenging activity (FRSA) with a half-inhibiting concentration (IC₅₀) value of 22.86 to 25.0 µg/mL, nitric oxide radical scavenging activity (NORSA) 141.3 to 149.1 µg/mL and for lipid peroxidation inhibition (LPI) 125 to 157.9 µg/mL. Usnic acid or Psoromic acid showed antioxidative action with IC₅₀ values ranging from 0.174 to 0.271 mg/mL. Methanol and ethyl acetate extract showed hydroxy-3-methyl-glutaryl-CoA reductase (HMGR) inhibition of 65.18 to 74.81%. Only 43.47% inhibition of angiotensin converting enzyme (ACE) was shown by methanol extract. Usnic acid showed noncompetitive type of HMGR inhibition and uncompetitive type of ACE inhibition. Psoromic acid exhibited competitive type of HMGR inhibition and mixed type of ACE inhibition.
CONCLUSIONS:
U. complanata showed both cardiovascular-protective and antioxidant properties. The lichen species U. complanata may be a natural bioresource for possible pharmaceutical applications.
Journal of Clinical Medicine, 2018, 7(8):226.
Antimycobacterial, Enzyme Inhibition, and Molecular Interaction Studies of Psoromic Acid in Mycobacterium tuberculosis: Efficacy and Safety Investigations.[Reference:
WebLink]
The current study explores the antimycobacterial efficacy of lichen-derived Psoromic acid (PA) against clinical strains of Mycobacterium tuberculosis (M.tb). Additionally, the inhibitory efficacy of PA against two critical enzymes associated with M.tb, namely, UDP-galactopyranose mutase (UGM) and arylamine-N-acetyltransferase (TBNAT), as drug targets for antituberculosis therapy were determined.
METHODS AND RESULTS:
PA showed a profound inhibitory effect towards all the M.tb strains tested, with minimum inhibitory concentrations (MICs) ranging between 3.2 and 4.1 µM, and selectivity indices (SIs) ranging between 18.3 and 23.4. On the other hand, the standard drug isoniazid (INH) displayed comparably high MIC values (varying from 5.4 to 5.8 µM) as well as low SI values (13.0–13.9). Interestingly, PA did not exhibit any cytotoxic effects on a human liver hepatocellular carcinoma cell line even at the highest concentration tested (75 µM). PA demonstrated remarkable suppressing propensity against UGM compared to standard uridine-5'-diphosphate (UDP), with 85.8 and 99.3% of inhibition, respectively. In addition, PA also exerted phenomenal inhibitory efficacy (half maximal inhibitory concentration (IC50) value = 8.7 µM, and 77.4% inhibition) against TBNAT compared with standard INH (IC50 value = 6.2 µM and 96.3% inhibition).
CONCLUSIONS:
Furthermore, in silico analysis validated the outcomes of in vitro assays, as the molecular interactions of PA with the active sites of UGM and TBNAT were unveiled using molecular docking and structure–activity relationship studies. Concomitantly, our findings present PA as an effective and safe natural drug plausible for use in controlling tuberculosis infections.
Fitoterapia, 2017, 121:164-169.
Antibacterial activities of natural lichen compounds against Streptococcus gordonii and Porphyromonas gingivalis.[Reference:
WebLink]
The oral bacteria not only infect the mouth and reside there, but also travel through the blood and reach distant body organs. If left untreated, the dental biofilm that can cause destructive inflammation in the oral cavity may result in serious medical complications. In dental biofilm, Streptococcus gordonii, a primary oral colonizer, constitutes the platform on which late pathogenic colonizers like Porphyromonas gingivalis, the causative agent of periodontal diseases, will bind. The aim of this study was to determine the antibacterial activity of eleven natural lichen compounds belonging to different chemical families and spanning from linear into cyclic and aromatic structures to uncover new antibiotics which can fight against the oral bacteria.
METHODS AND RESULTS:
The compounds were screened by broth microdilution assay. Three compounds were shown to have promising antibacterial activities where the depsidone core with certain functional groups constituted the best compound, Psoromic acid, with the lowest MICs=11.72 and 5.86μg/mL against S. gordonii and P. gingivalis, respectively.
CONCLUSIONS:
The compounds screened had promising antibacterial activity which might be attributed to some important functional groups as discussed in our study. The best compounds did not induce the death of gingival epithelial carcinoma cells (Ca9-22). These results introduce new compounds having potent antibacterial activities against oral pathogens causing serious medical complications.
Journal of the American Chemical Society, 2012, 134(17):p.7384-7391.
Psoromic acid is a selective and covalent Rab-prenylation inhibitor targeting autoinhibited RabGGTase.[Reference:
WebLink]
Post-translational attachment of geranylgeranyl isoprenoids to Rab GTPases, the key organizers of intracellular vesicular transport, is essential for their function. Rab geranylgeranyl transferase (RabGGTase) is responsible for prenylation of Rab proteins. Recently, RabGGTase inhibitors have been proposed to be potential therapeutics for treatment of cancer and osteoporosis. However, the development of RabGGTase selective inhibitors is complicated by its structural and functional similarity to other protein prenyltransferases.
METHODS AND RESULTS:
Herein we report identification of the natural product Psoromic acid (PA) that potently and selectively inhibits RabGGTase with an IC(50) of 1.3 μM. Structure-activity relationship analysis suggested a minimal structure involving the depsidone core with a 3-hydroxyl and 4-aldehyde motif for binding to RabGGTase. Analysis of the crystal structure of the RabGGTase:PA complex revealed that PA forms largely hydrophobic interactions with the isoprenoid binding site of RabGGTase and that it attaches covalently to the N-terminus of the α subunit. We found that in contrast to other protein prenyltransferases, RabGGTase is autoinhibited through N-terminal (α)His2 coordination with the catalytic zinc ion. Mutation of (α)His dramatically enhances the reaction rate, indicating that the activity of RabGGTase is likely regulated in vivo. The covalent binding of PA to the N-terminus of the RabGGTase α subunit seems to potentiate its interaction with the active site and explains the selectivity of PA for RabGGTase.
CONCLUSIONS:
Therefore, Psoromic acid provides a new starting point for the development of selective RabGGTase inhibitors.
Alternatives to laboratory animals atla, 2004, 32(6):605.
Cytotoxic and apoptotic effects on hepatocytes of secondary metabolites obtained from lichens.[Reference:
WebLink]
There are a large number of species of Antarctic lichens, and several studies describing the secondary metabolites present in these lichens, as well as the advances in understanding the chemistry of these metabolites, have been reported. In addition, some derivatives displaying interesting antibacterial effects have been described.
METHODS AND RESULTS:
The cytotoxic and apoptotic effects of 15 secondary metabolites (depsides, depsidones and usnic acid) obtained from Continental (Chilean) and Antarctic lichens were evaluated in primary cultures of rat hepatocytes. Intracellular lactate dehydrogenase release, caspase 3 activation and DNA fragmentation were measured. In this study, we have evaluated a set of markers associated with pivotal steps in the execution phase of apoptosis, in order to detect compounds with apoptotic effects on hepatocytes before significant necrosis takes place.
CONCLUSIONS:
Flow cytometric analysis of DNA fragmentation revealed an increase in apoptotic nuclei with sub-diploid DNA content after the exposure of hepatocytes to sub-cytotoxic concentrations of the compounds. Among these, salazinic acid, stictic acid and Psoromic acid displayed significant apoptotic activities.