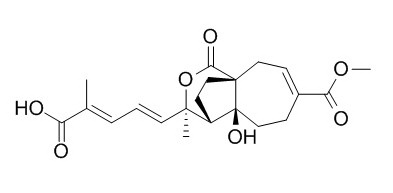
Pseudolaric Acid C
Pseudolaric Acid C shows weak antifungal activity against Candida albicans.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Mol Divers.2022, s11030-022-10586-3.
Theranostics.2023, 13(9):3103-3116.
Horticulturae2022, 8(10), 975.
J Med Food.2021, 24(2):151-160.
AMB Express2020. 10(1):126.
JAOCS2021, 98(7):779-794.
African J. Agricultural Research 2017, 12(13):1164-1168
Front Pharmacol.2022, 13:906763.
Agriculture.2024, 69(3):140-148.
BioRxiv-The Preprint server for biology2023, 586957.
Related and Featured Products
Chinese Traditional & Herbal Drugs, 2012 , 43 (2) :220-222.
Antifungal constituents of Pseudolaricis Cortex.[Reference:
WebLink]
To study the chemical constituents from Pseudolaricis Cortex with antifungal activities.
METHODS AND RESULTS:
The crude powder of Pseudolaricis Cortex was refluxed with 95% ethanol and different fractions were obtained with different solvants. In vitro antifungal results revealed that EtOAc fraction showed inhibitory effects on Candida albicans. Compounds were isolated by column chromatography on silica gel, Sephadex LH-20, and preparative HPLC from EtOAc fraction of Pseudolaricis Cortex and their structures were elucidated mainly by spectral data. Five compounds were identified as pseudolaric acid B (1), Pseudolaric Acid C (2), vanillic acid (3), vanillic acid-4-O-β-D-allopyranoside (4), and 17-hydroxypseudolaric acid B (5), respectively.
CONCLUSIONS:
Compound 5 is a new compound named pseudolaric acid I, and compound 4 is isolated from this plant for the first time. Compound 1 shows stronger activity against the growth of Candida albicans, while compounds 2 and 5 have weak antifungal activities in vitro.
J Nat Prod. 1999 May;62(5):767-9.
Two auronols from Pseudolarix amabilis.[Pubmed:
10346966 ]
Two new auronols, amaronols A (1) and B (2), were isolated from the bark of Pseudolarix amabilis, along with pseudolaric acid B (3), Pseudolaric Acid C (4), demethoxydeacetoxy-pseudolaric acid B (5), pseudolaric acid B-beta-D-glucoside (6), pseudolaric acid A-beta-D-glucoside (7), and myricetin (8).
METHODS AND RESULTS:
The structures of amaronols A and B were established by spectral data interpretation as 2,4,6-trihydroxy-2-[(3',4',5'-trihydroxyphenyl) methyl]-3(2H)-benzofuranone and 2,4,6-trihydroxy-2-[(3', 5'-dihydroxy-4'-methoxyphenyl) methyl]-3(2H)-benzofuranone, respectively. Antimicrobial testing results of the eight compounds indicated that only pseudolaric acid B was active against Candida albicans (MIC, 3.125 microg/mL; MFC, 6.25 microg/mL), while myricetin was marginally active against Trichophyton mentagrophytes (MIC, 50 microg/mL).