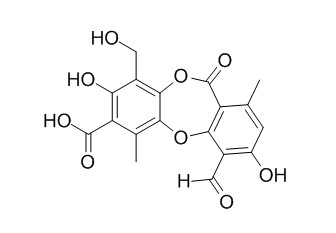
Protocetraric acid
Protocetraric acid demonstrates a strong antioxidant, antimicrobial, and anticancer effects.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2024, 29(6):1240.
J Ginseng Res.2023, 47(4):593-603.
Biomed Chromatogr.2020, e5021.
Cell J.2024, 26(8):496-504.
J Pain Res.2022, 15:3469-3478.
J Nat Prod.2021, 84(9):2544-2553.
Food Engineering Progress2019, 23(3)209-216
PLoS One.2017, 12(3):e0173585
Food Chem X.2024, 24:101989.
Korean Journal of Medicinal Crop Science2018, 26(5):382-390
Related and Featured Products
Planta Medica, 2008, 74(9):996-996.
Antimicrobial activity of fumarprotocetraric acid, lecanoric acid, protocetraric acid and stictic acid isolated from different species of lichen.[Reference:
WebLink]
The antimicrobial activity of fumarProtocetraric acid, lecanoric acid, Protocetraric acid and stictic acid isolated from the lichens Cladonia furcata (Huds.) Schrad., Ochrolechia androgyna (Hoffm.) Arn., Parmelia caperata (L.) Ach. and Parmelia conspresa (Ach.) Ach. was studied for the following microorganisms: Bacillus mycoides, Bacillus subtilis, Staphylococcus aureus, Enterobacter cloaceae, Escherichia coli, Klebsiella pneumoniae, Aspergillus flavus (ATCC 9170), Aspergillus fumigatus, Botrytis cinerea, Candida albicans, Fusarium oxysporum, Mucor mucedo (ATCC 52568), Paecilomyces variotii (ATCC 22319), Penicillium purpurescens, Penicillium verrucosum, Trichoderma harsianum. Aspergillus flavus, Mucor mucedo and Paecilomyces variotii are ATCC type, and the other microorganisms are clinical isolates.
METHODS AND RESULTS:
The antimicrobal activity was estimated by determining the minimum inhibitory concentration (MIC) by the Broth tube Dilution method. The tested lichen components ihibited growth of all the tested microrganisms. The bacteria showed a bigger sensitivity relative to fungi. The lowest MIC value (0.031mg/mL) was measured for the fumarProtocetraric acid related to the Klebsiella pneumoniae species. The weakest antimicrobial activity was found in stictic acid, which inhibited most of the tested microorganisms in significantly higher concentrations.
CONCLUSIONS:
Generally, all the components had relatively strong antimicrobial activity against the tested microorganisms.
Phytomedicine, 2012, 19(13):1166-1172.
Chemical composition of three Parmelia lichens and antioxidant, antimicrobial and cytotoxic activities of some their major metabolites.[Reference:
WebLink]
The aim of this study is to investigate chemical composition of acetone extracts of the lichens Parmelia caperata, P. saxatilis and P. sulcata and antioxidant, antimicrobial and anticancer activities of some their major metabolites.
METHODS AND RESULTS:
The phytochemical analysis of acetone extracts of three Parmelia lichens were determined by HPLC-UV method. The predominant phenolic compounds in these extracts were Protocetraric acid and usnic acid (P. caperata) and depsidone salazinic acid (other two species). Besides these compounds, atranorin and chloroatranorin, were also detected in some of these extracts. Antioxidant activity of their isolated metabolites was evaluated by free radical scavenging, superoxide anion radical scavenging and reducing power. As a result of the study salazinic acid had stronger antioxidant activity than Protocetraric acid. The antimicrobial activity was estimated by determination of the minimal inhibitory concentration by the broth microdilution method. Both compounds were highly active with minimum inhibitory concentration values ranging from 0.015 to 1 mg/ml. Anticancer activity was tested against FemX (human melanoma) and LS174 (human colon carcinoma) cell lines using MTT method. Salazinic acid and Protocetraric acid were found to be strong anticancer activity toward both cell lines with IC50 values ranging from 35.67 to 60.18 μg/ml.
CONCLUSIONS:
The present study shows that tested lichen compounds demonstrated a strong antioxidant, antimicrobial, and anticancer effects. That suggest that these lichens can be used as new sources of the natural antimicrobial agents, antioxidants and anticancer compounds.