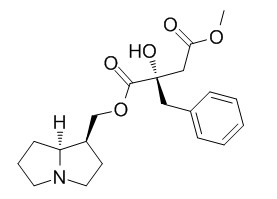
Phalaenopsine Is
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Ethnopharmacol.2019, 228:132-141
Mol Pharm.2017, 14(9):3164-3177
Journal of Food Quality2022, P:13, 6256310.
J Ethnopharmacol.2020, 249:112381
Front Endocrinol (Lausanne).2020, 11:568436.
Horticulturae2024, 10(4), 382.
Separations2023, 10(4),255.
Molecules.2019, 24(22):E4022
Phytomedicine.2019, 58:152893
Asian Pac J Cancer Prev.2019, 20(1):65-72
Related and Featured Products
Phytochemistry (Amsterdam), 2006, 67(14):1493-1502.
Tissue distribution and biosynthesis of 1,2-saturated pyrrolizidine alkaloids in Phalaenopsis hybrids (Orchidaceae).[Reference:
WebLink]
Phalaenopsis hybrids contain two 1,2-saturated pyrrolizidine monoesters, T-phalaenopsine (necine base trachelanthamidine) and its stereoisomer Is-phalaenopsine (necine base isoretronecanol).
T-Phalaenopsine Is the major alkaloid accounting for more than 90% of total alkaloid. About equal amounts of alkaloid were genuinely present as free base and its N-oxide.
METHODS AND RESULTS:
The structures were confirmed by GC–MS. The quantitative distribution of phalaenopsine in various organs and tissues of vegetative rosette plants and flowering plants revealed alkaloid in all tissues. The highest concentrations were found in young and developing tissues (e.g., root tips and young leaves), peripheral tissues (e.g., of flower stalks) and reproductive organs (flower buds and flowers). Within flowers, parts that usually attract insect visitors (e.g., labellum with colorful crests as well as column and pollinia) show the highest alkaloid levels. Tracer feeding experiments with 14C-labeled putrecine revealed that in rosette plants the aerial roots were the sites of phalaenopsine biosynthesis. However active biosynthesis was only observed in roots still attached to the plant but not in excised roots. There is a slow but substantial translocation of newly synthesized alkaloid from the roots to other plant organs. A long-term tracer experiment revealed that phalaenopsine shows neither turnover nor degradation.
CONCLUSIONS:
The results are discussed in the context of a polyphyletic molecular origin of the biosynthetic pathways of pyrrolizidine alkaloids in various scattered angiosperm taxa. The ecological role of the so called non-toxic 1,2-saturated pyrrolizidine alkaloids is discussed in comparison to the pro-toxic 1,2-unsaturated pyrrolizidine alkaloids. Evidence from the plant-insect interphase is presented indicating a substantial role of the 1,2-saturated alkaloids in plant and insect defense.