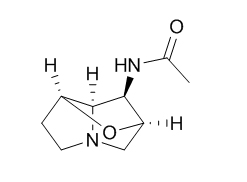
N-Acetylnorloline
High levels of N-acetyl norloline and peramine may be of particular importance for developing 'friendly' endophyte-enhanced turf and pasture grasses that resist challenging lepidopteran pests. N-acetyl norloline, which is produced by the Max P endophyte, may be responsible for a new form of toxicity called equine fescue oedema in horses.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Pharmaceuticals.2022, 15(4), 402.
Research on Crops.2017, 18(3):569
Evid Based Complement Alternat Med.2018, 2018:4580627
Food Res Int.2022, 157:111207.
J Pharm Biomed Anal.2022, 207:114398.
Neuropharmacology2019, 151437
Journal of Functional Foods2022, 91:105019.
Molecules.2020, 25(18),4089.
Chem Biol Interact.2019, 315:108910
Molecules.2019, 24(19):E3417
Related and Featured Products
PLoS One. 2014 Dec 22;9(12):e115590.
Enzymes from fungal and plant origin required for chemical diversification of insecticidal loline alkaloids in grass-Epichloë symbiota.[Pubmed:
25531527]
The lolines are a class of bioprotective alkaloids that are produced by Epichloë species, fungal endophytes of grasses. These alkaloids are saturated 1-aminopyrrolizidines with a C2 to C7 ether bridge, and are structurally differentiated by the various modifications of the 1-amino group: -NH2 (norloline), -NHCH3 (loline), -N(CH3)2 (N-methylloline), -N(CH3)Ac (N-acetylloline), -NHAc (N-Acetylnorloline), and -N(CH3)CHO (N-formylloline). Other than the LolP cytochrome P450, which is required for conversion of N-methylloline to N-formylloline, the enzymatic steps for loline diversification have not yet been established.
METHODS AND RESULTS:
Through isotopic labeling, we determined that N-Acetylnorloline is the first fully cyclized loline alkaloid, implying that deacetylation, methylation, and acetylation steps are all involved in loline alkaloid diversification. Two genes of the loline alkaloid biosynthesis (LOL) gene cluster, lolN and lolM, were predicted to encode an N-acetamidase (deacetylase) and a methyltransferase, respectively. A knockout strain lacking both lolN and lolM stopped the biosynthesis at N-Acetylnorloline, and complementation with the two wild-type genes restored production of N-formylloline and N-acetylloline. These results indicated that lolN and lolM are required in the steps from N-Acetylnorloline to other lolines. The function of LolM as an N-methyltransferase was confirmed by its heterologous expression in yeast resulting in conversion of norloline to loline, and of loline to N-methylloline. One of the more abundant lolines, N-acetylloline, was observed in some but not all plants with symbiotic Epichloë siegelii, and when provided with exogenous loline, asymbiotic meadow fescue (Lolium pratense) plants produced N-acetylloline, suggesting that a plant acetyltransferase catalyzes N-acetylloline formation.
CONCLUSIONS:
We conclude that although most loline alkaloid biosynthesis reactions are catalyzed by fungal enzymes, both fungal and plant enzymes are responsible for the chemical diversification steps in symbio.
Environ. Entomol., 2011, 40(3):639-47.
Endophyte-mediated resistance to black cutworm as a function of plant cultivar and endophyte strain in tall fescue.[Pubmed:
22251642]
METHODS AND RESULTS:
To improve Neotyphodium endophyte-mediated resistance to black cutworm Agrotis ipsilon (Hufnagel) (BCW), a series of experiments was conducted by using several different cultivars of tall fescue, Schedonorus arundinaceus (Schreb.) Dumort. in combination with several different haplotypes of the endophyte Neotyphodium coenophialum (Morgan-Jones & Gams) (plant cultivar × endophyte haplotype = plant line), each producing unique alkaloid profiles.
BCW settling response, survival at 5 and 10 d, and larval biomass varied significantly among plant lines. In general, greater variation BCW performance was observed within a single plant cultivar infected with different endophyte haplotypes than among different plant cultivars infected with the same endophyte haplotype, but comparisons among the former were far more numerous. Although five endophyte-mediated alkaloids representing three alkaloid classes were quantified in the plants, the pyrrolizidine alkaloid N-acetyl norloline was consistently the single best predictor of BCW performance. BCW settling response, 5-d survival, and 10-d survival decreased as levels of the alkaloid N-acetyl norloline increased. The same three response variables also decreased with increasing levels of peramine, but increased with increasing levels of ergovaline. Minor variation in endophyte infection levels occurring among infected plant lines had no significant influence on BCW performance.
CONCLUSIONS:
Results indicate a potentially important role for N-acetyl norloline and peramine in providing resistance to black cutworm whereas ergovaline appears to be much less important. Therefore, endophyte haplotypes expressing high levels of N-acetyl norloline and peramine may be of particular importance for developing 'friendly' endophyte-enhanced turf and pasture grasses that resist challenging lepidopteran pests, although remaining safe for wildlife and grazing mammals.
Aust Vet J. 2009 Dec;87(12):492-8.
Fescue-associated oedema of horses grazing on endophyte-inoculated tall fescue grass (Festuca arundinacea) pastures.[Pubmed:
19930166 ]
A new form of toxicity called equine fescue oedema is described.
METHODS AND RESULTS:
The clinical signs included inappetence, depression, and subcutaneous oedema of the head, neck, chest and abdomen. Affected horses had very low plasma albumin values.
The toxicity affected 48 of 56 horses on six farms in different states of Australia, and 4 horses have died. All horses were grazing pastures that had been sown with varieties of Mediterranean tall fescue (Festuca arundinacea) that carry the endophyte known as Max P or Max Q.
CONCLUSIONS:
It is proposed that a pyrrolizidine alkaloid, N-Acetylnorloline, which is produced by the Max P endophyte, may be responsible for this new toxicity in horses.
738/5000
Phytochemistry. 2014 Feb;98:60-8.
Ether bridge formation in loline alkaloid biosynthesis.[Pubmed:
24374065]
Lolines are potent insecticidal agents produced by endophytic fungi of cool-season grasses. These alkaloids are composed of a pyrrolizidine ring system and an uncommon ether bridge linking carbons 2 and 7. Previous results indicated that 1-aminopyrrolizidine was a pathway intermediate.
METHODS AND RESULTS:
We used RNA interference to knock down expression of lolO, resulting in the accumulation of an alkaloid identified as exo-1-acetamidopyrrolizidine based on high-resolution MS and NMR. Genomes of endophytes differing in alkaloid profiles were sequenced, revealing that those with mutated lolO accumulated exo-1-acetamidopyrrolizidine but no lolines. Heterologous expression of wild-type lolO complemented a lolO mutant, resulting in the production of N-Acetylnorloline.
CONCLUSIONS:
These results indicated that the non-heme iron oxygenase, LolO, is required for ether bridge formation, probably through oxidation of exo-1-acetamidopyrrolizidine.