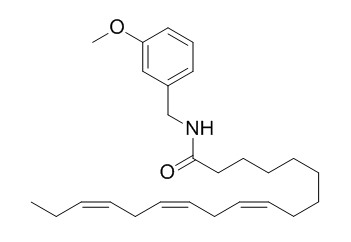
N-(3-Methoxybenzyl)(9Z,12Z,15Z)-octadeca-9,12,15-trienamide
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J.Pharm. & Biome. Anal.2023, 2: 100018.
Front Endocrinol (Lausanne).2023, 14:1138676.
Molecules.2021, 26(6):1635.
Biomed Pharmacother.2020, 125:109784.
Anticancer Res.2020, 40(10):5529-5538.
Antioxidants (Basel).2020, 9(2):E120
Antioxidants (Basel).2022, 11(12):2496.
Oncotarget.2017, 9(3):4161-4172
Int J Mol Sci.2020, 21(19):7209.
J Traditional Thai Medical Res.2022, 8(1):pp1-14.
Related and Featured Products
Journal of Natural Products, 2014, 77(7):1663-1669.
Identification of Endocannabinoid System-Modulating N-Alkylamides from Heliopsis helianthoides var. scabra and Lepidium meyenii[Reference:
WebLink]
The discovery of the interaction of plant-derived N-alkylamides (NAAs) and the mammalian endocannabinoid system (ECS) and the existence of a plant endogenous N-acylethanolamine signaling system have led to the re-evaluation of this group of compounds.
METHODS AND RESULTS:
Herein, the isolation of seven NAAs and the assessment of their effects on major protein targets in the ECS network are reported. Four NAAs,
octadeca-2E,4E,8E,10Z,14Z-pentaene-12-ynoic acid isobutylamide (1), octadeca-2E,4E,8E,10Z,14Z-pentaene-12-ynoic acid 2'-methylbutylamide (2), hexadeca-2E,4E,9Z-triene-12,14-diynoic acid isobutylamide (3), and hexadeca-2E,4E,9,12-tetraenoic acid 2'-methylbutylamide (4), were identified from Heliopsis helianthoides var. scabra. Compounds 2-4 are new natural products, while 1 was isolated for the first time from this species. The previously described macamides, N-(3-methoxybenzyl)-(9Z,12Z,15Z)-octadecatrienamide (N-(3-Methoxybenzyl)(9Z,12Z,15Z)-octadeca-9,12,15-trienamide, 5), N-benzyl-(9Z,12Z,15Z)-octadecatrienamide (6), and N-benzyl-(9Z,12Z)-octadecadienamide (7), were isolated from Lepidium meyenii (Maca). N-Methylbutylamide 4 and N-benzylamide 7 showed submicromolar and selective binding affinities for the cannabinoid CB1 receptor (Ki values of 0.31 and 0.48 μM, respectively). Notably, compound 7 also exhibited weak fatty acid amide hydrolase (FAAH) inhibition (IC50 = 4 μM) and a potent inhibition of anandamide cellular uptake (IC50 = 0.67 μM) that was stronger than the inhibition obtained with the controls OMDM-2 and UCM707. The pronounced ECS polypharmacology of compound 7 highlights the potential involvement of the arachidonoyl-mimicking 9Z,12Z double-bond system in the linoleoyl group for the overall cannabimimetic action of NAAs.
CONCLUSIONS:
This study provides additional strong evidence of the endocannabinoid substrate mimicking of plant-derived NAAs and uncovers a direct and indirect cannabimimetic action of the Peruvian Maca root.