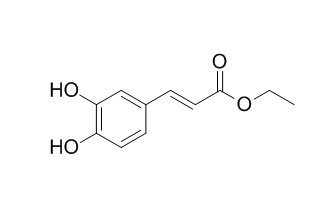
Ethyl caffeate
Ethyl caffeate is a potent chemopreventive compound against skin carcinogenesis caused by solar UV exposure, it strongly inhibits neoplastic transformation of JB6 Cl41 cells without toxicity. PI3K, ERK1/2, and p38 kinase activities were suppressed by direct binding with HOEC in vitro. Ethyl caffeate also has anti-inflammatory, hepatprotective activities.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J of Dentistry & Oral Health2019, 2641-1962
Nutrients.2024, 16(7):985.
Asian Journal of Chemistry2014, 26(8):2425
Pharmaceuticals (Basel).2020, 13(10):302.
Cancer Lett. 2023, 18:216584.
BMC Plant Biol.2022, 22(1):128.
Plant J.2021, 107(6):1711-1723.
Antioxidants (Basel).2022, 11(12):2496.
Antioxidants (Basel).2020, 9(6):544.
Food and Fermentation Industries2019, 45(7):45-51
Related and Featured Products
Chem Biol Interact. 2014 Aug 5;219:151-8.
A mechanistic study on the anti-cancer activity of ethyl caffeate in human ovarian cancer SKOV-3 cells.[Pubmed:
24892518 ]
In the present study, we investigated the effect and molecular mechanism of Ethyl caffeate (EC), a natural phenolic compound isolated from Ligularia fischeri, on human ovarian cancer cell proliferation and progression.
METHODS AND RESULTS:
EC-mediated inhibition of cell proliferation in SKOV-3 cells was accompanied by reduced expression of cell cycle-related proteins such as cyclin-dependent kinases and cyclins, resulting in pRb hypophosphorylation and G₁ phase cell cycle arrest. Moreover, EC treatment markedly inhibited cell migration and invasion. These regulatory effects of EC on ovarian cancer cell proliferation, migration and invasion were associated with inactivation of mitogenic signaling pathways such as Akt, ERK and p38(MAPK), and down-regulation of cell surface signaling molecules including receptor tyrosine kinases, integrin α3β1 and N-cadherin.
CONCLUSIONS:
Taken together, these findings suggest further evaluation and development of EC for the treatment and prevention of ovarian cancer.
Br J Pharmacol. 2005 Oct;146(3):352-63.
Ethyl caffeate suppresses NF-kappaB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2 in vitro or in mouse skin.[Pubmed:
16041399]
Ethyl caffeate, a natural phenolic compound, was isolated from Bidens pilosa, a medicinal plant popularly used for treating certain inflammatory syndromes. The purpose of this study was to investigate the structural activity, and the anti-inflammatory functions and mechanism(s) of Ethyl caffeate.
METHODS AND RESULTS:
Ethyl caffeate was found to markedly suppress the lipopolysaccharide (LPS)-induced nitric oxide (NO) production (IC(50) = 5.5 microg ml(-1)), mRNA and protein expressions of inducible nitric oxide synthase (iNOS), and prostaglandin E(2) (PGE(2)) production in RAW 264.7 macrophages. Transient gene expression assays using human cox-2 promoter construct revealed that Ethyl caffeate exerted an inhibitory effect on cox-2 transcriptional activity in 12-O-tetradecanoylphorbol-13-acetate (TPA)-treated MCF-7 cells. Immunohistochemical studies of mouse skin demonstrated that TPA-induced COX-2 expression was significantly inhibited by Ethyl caffeate with a superior effect to that of celecoxib, a nonsteroidal anti-inflammatory drug. The phosphorylation and degradation of inhibitor kappaB (IkappaB) and the translocation of nuclear transcription factor-kappaB (NF-kappaB) into the nucleus, as well as the activation of mitogen-activated protein kinases (MAPKs) induced by LPS in macrophages, were not affected by Ethyl caffeate. Ethyl caffeate, however, could inhibit NF-kappaB activation by impairing the binding of NF-kappaB to its cis-acting element. These results suggest that Ethyl caffeate suppresses iNOS and COX-2 expressions partly through the inhibition of the NF-kappaB.DNA complex formation. Structure-activity relationship analyses suggested that the catechol moiety and alpha,beta-unsaturated ester group in Ethyl caffeate are important and essential structural features for preventing NF-kappaB.DNA complex formation.
CONCLUSIONS:
This study provides an insight into the probable mechanism(s) underlying the anti-inflammatory and therapeutic properties of Ethyl caffeate.
J Sep Sci. 2009 Nov;32(21):3585-90.
Ethyl caffeate from Verdicchio wine: chromatographic purification and in vivo evaluation of its antifibrotic activity.[Pubmed:
19813225]
Ethyl caffeate (CfE, caffeic acid ethyl ester) was extracted from dealcoholized Verdicchio, a white wine from Marche (Italy) with ethyl acetate and then purified with semipreparative reversed-phase high-performance liquid chromatography (RP-HPLC) using an ODS2 column (25 cm x 20 mm id) at an isocratic flow of 5 mL/min (the mobile phase A was formic acid 4.5% in water and the mobile phase B was acetonitrile).
METHODS AND RESULTS:
The CfE extract administered intraperitoneally at 1 mumol/L in rats previously treated with 10 mg/kg dimethylnitrosamine was able to prevent the dimethylnitrosamine-induced loss in body and liver weight, as well as to reduce the degree of liver injury, as determined by alanine aminotransferase values and necroinflammatory score, after a 1-week treatment. This was associated with a reduced hepatic stellate cells activation (from 16.8 to 8.3% of smooth muscle actin positive parenchyma) and proliferation (from 11.3 to 5.5 cells/mm(2)). The collagen synthesis was also reduced: the percentage of Sirius Red positive parenchyma decreased from 21.7 to 7.2%.
CONCLUSIONS:
The CfE levels of Verdicchio wine determined with RP-HPLC-DAD were about 14 times the active levels tested in the in vivo test. CfE can be considered as a promising natural compound for future application in chronic liver disease.
Cancer Prev Res (Phila). 2014 Aug;7(8):856-65.
(+)-2-(1-Hydroxyl-4-oxocyclohexyl) ethyl caffeate suppresses solar UV-induced skin carcinogenesis by targeting PI3K, ERK1/2, and p38.[Pubmed:
24845061]
For decades, skin cancer incidence has increased, mainly because of oncogenic signaling pathways activated by solar ultraviolet (UV) irradiation (i.e., sun exposure). Solar UV induces multiple signaling pathways that are critical in the development of skin cancer, and therefore the development of compounds capable of targeting multiple molecules for chemoprevention of skin carcinogenesis is urgently needed.
METHODS AND RESULTS:
Herein, we examined the chemopreventive effects and the molecular mechanism of (+)-2-(1-hydroxyl-4-oxocyclohexyl) Ethyl caffeate (HOEC), isolated from Incarvillea mairei var. grandiflora (Wehrhahn) Grierson. HOEC strongly inhibited neoplastic transformation of JB6 Cl41 cells without toxicity. PI3K, ERK1/2, and p38 kinase activities were suppressed by direct binding with HOEC in vitro. Our in silico docking data showed that HOEC binds at the ATP-binding site of each kinase. The inhibition of solar UV-induced PI3K, ERK1/2, and p38 kinase activities resulted in suppression of their downstream signaling pathways and AP1 and NF-κB transactivation in JB6 cells. Furthermore, topical application of HOEC reduced skin cancer incidence and tumor volume in SKH-1 hairless mice chronically exposed to solar UV.
CONCLUSIONS:
In summary, our results show that HOEC exerts inhibitory effects on multiple kinase targets and their downstream pathways activated by solar UV in vitro and in vivo. These findings suggest that HOEC is a potent chemopreventive compound against skin carcinogenesis caused by solar UV exposure.
J Med Chem. 2012 Nov 26;55(22):10177-86.
Order and disorder: differential structural impacts of myricetin and ethyl caffeate on human amylase, an antidiabetic target.[Pubmed:
23050660]
The increasing prevalence of diabetes has accelerated the search for new drugs derived from natural sources. To define the functional features of two such families of compounds, the flavonols and the Ethyl caffeates, we have determined the high-resolution structures of representative inhibitors in complex with human pancreatic α-amylase.
METHODS AND RESULTS:
Myricetin binds at the active site and interacts directly with the catalytic residues despite its bulky planar nature. Notably, it reduces the normal conformational flexibility of the adjacent substrate binding cleft. In contrast, bound Ethyl caffeate acts by disordering precisely those polypeptide chain segments that make up the active site binding cleft. It also operates from binding sites far removed from the active site, a property not observed in any other class of human α-amylase inhibitor studied to date.
CONCLUSIONS:
Given the current inadequacy of drugs directed at diabetes, the use of optimized flavonols and Ethyl caffeates may present an alternative therapeutic route.