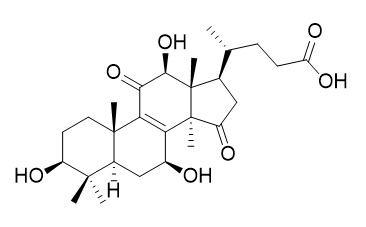
Lucidenic acid C
Lucidenic acid C has anti-invasive effect, it shows significant inhibitory effects on PMA-induced MMP-9 activity and invasion of HepG(2 )cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Cosmet Sci.2019, 41(1):12-20
Hong Kong Baptist University2023, 048330T.
J Microbiol Immunol Infect.2021, S1684-1182(21)00142-0.
HortTechnology2016, 26(6):816-819
Molecules.2022, 27(7):2360.
J Appl Microbiol.2024, 135(7):lxae180.
J Sep Sci.2020, 201901140
Food Science and Preservation2024, 31(3):486-498.
Free Radic Biol Med.2016, 97:307-319
Virulence.2018, 9(1):588-603
Related and Featured Products
Mol Nutr Food Res. 2007 Dec;51(12):1472-7.
The anti-invasive effect of lucidenic acids isolated from a new Ganoderma lucidum strain.[Pubmed:
17979098 ]
Ganoderma lucidum is a well-known mushroom with various pharmacological effects that has been used for health and longevity purposes. The objective of this study was to investigate the anti-invasive effect of lucidenic acids isolated from a new G. lucidum strain (YK-02) against human hepatoma carcinoma (HepG(2)) cells.
METHODS AND RESULTS:
Triterpenoid components in the ethanol extract of G. lucidum (YK-02) were separated by means of a semi-preparative RP HPLC. Four major peaks were separated and crystallized from triterpenoids fraction, and were identified as lucidenic acid A, lucidenic acid B, Lucidenic acid C, and lucidenic acid N according to their spectroscopic values of (1)H NMR and MS. Treatment of the lucidenic acids (50 microM) in the presence of 200 nM phorbol 12-myristate 13-acetate (PMA) after 24 h of incubation all resulted in significant inhibitory effects on PMA-induced MMP-9 activity and invasion of HepG(2 )cells.
CONCLUSIONS:
The results indicate that the lucidenic acids isolated from G. lucidum (YK-02) are anti-invasive bioactive components on hepatoma cells.
Yao Xue Xue Bao. 2001 Aug;36(8):595-8.
A new triterpene from the fruiting bodies of Ganoderma lucidum.[Pubmed:
12579936]
To study the chemical constituents of the fruiting bodies of Ganoderma lucidum.
METHODS AND RESULTS:
Individual constituents, isolated and repeatedly purified on silica gel column, were identified by physicochemical constants and structurally elucidated by spectral methods.
From the alcohol extract, compound 2 was obtained and identified as 3 beta,7 beta-dihydroxy-4,4,14 alpha-trimethyl-11,15-dioxo-5 alpha-chol-8-en-24-oic acid. In addition, two known compounds, lucidenic acid A (1) and C (3) were obtained.
CONCLUSIONS:
Compound 2 is a new triterpene compound.
Zhongguo Zhong Yao Za Zhi. 2017 May;42(10):1908-1915.
Ganoderma triterpenoids from aqueous extract of Ganoderma lucidum.[Pubmed:
29090550]
METHODS AND RESULTS:
A new triterpenoid and 18 analogues were isolated from the water extract of Ganoderma lucidum by column chromatographic techniques, including silica gel, ODS, Sephadex LH-20, and HPLC. The new compound was elucidated as 2β-acetoxy-3β,25-dihydroxy-7,11,15-trioxo-lanost-8-en-26-oic acid on the basis of analyses of extensive spectroscopic data and its physicochemical properties. Comparison of NMR data with those reported in literature, the known analogues were determined as ganoderic acid H (2), 12β-acetoxy-3β,7β-dihydroxy-11,15,23-trioxo-lanost-8,16-dien-26-oic acid (3), ganoderenic acid D (4),ganoderic acid C1 (5),ganoderic acid G (6),3β,7β-dihydroxy-11,15,23-trioxo-lanost-8,16-dien-26-oic acid (7),ganoderic acid B (8),ganoderic acid C6 (9),3β,15α-dihydroxy-7,11,23-trioxo-lanost-8,16-dien-26-oic acid (10),ganoderic acid A (11),ganolucidic acid A (12),lucidenic acid E2 (13),lucidenic acid N (14),lucidenic acid P (15), lucidenic acid B (16),lucidenic acid A (17),Lucidenic acid C (18),and lucidenic acid L (19), respectively.
CONCLUSIONS:
Compound 1 is new compound and compounds 2-19 have been reported from G. lucidum. The present study enriches the knowledge of the chemical constituent of G. lucidum and completes chemical investigation of water decoction that is traditional use of G. lucidum.