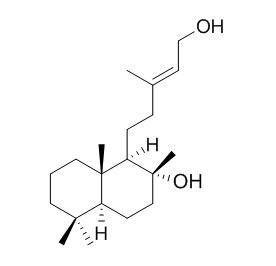
Labd-13-ene-8,15-diol
13(E)-Labd-13-ene-8alpha,15-diol shows antiviral and anticancer activity, it shows strong anti-HRV2 and HRV3 activity with a 50% inhibitory concentration (IC50) of 2.68 and 0.87 microg/mL,respectively; it also exhibits antilung and antilaryngeal cancer activities against A549 and Hep2 cells.13(E)-Labd-13-ene-8α,15-diol inhibits the growth of the gram-positive bacteria (Staphylococcus aureus, Bacillus cereus and Listeria monocytogenes) and gram-negative bacteria (Vibrio parahaemolyticus, Escherichia coli and Salmonella enteritidis) with a range of minimum inhibitory concentration (MIC) values from 0.092 to 0.598 mg/mL and gram-negative bacteria are more sensitive to the compound (MIC, 0.092 mg/mL).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Research International2016, 106-113
J Pharm Biomed Anal.2024, 247:116257.
Int J Biol Macromol.2018, 112:1093-1103
J Liq Chromatogr R T2025, 2505536.
Toxins (Basel).2019, 11(10):E575
Scientific World Journal.2014, 2014:654193
Chem Biol Interact.2020, 328:109200.
Vietnam J. Chem.2023, 61(3),308-317
J Food Drug Anal.2023, 31(2):254-277.
Korean Journal of Pharmacognosy2018, 49(4):349-361
Related and Featured Products
Phytother. Res.,2010 Feb;24(2):169-74.
Antiviral and anticancer activities of 13(E)-labd-13-ene-8alpha,15-diol isolated from Brachyglottis monroi.[Pubmed:
19504469 ]
METHODS AND RESULTS:
The antiviral activity of 13(E)-labd-13-ene-8alpha,15-diol (Labd-13-ene-8,15-diol,1), isolated from Brachyglottis monroi, was examined against human rhinovirus 2 (HRV2) and 3 (HRV3), and the anticancer activity on human cancer cells (A549 and Hep2). Compound (1) showed strong anti-HRV2 and HRV3 activity with a 50% inhibitory concentration (IC(50)) of 2.68 and 0.87 microg/mL, respectively, and a 50% cytotoxicity concentration (CC(50)) of 59.45 microg/mL. Ribavirin only showed anti-HRV3 activity with an IC(50) of 30.48 microg/mL and a CC(50) > 100 microg/mL. The addition of compound (1) to HRV-infected HeLa cells directly reduced the formation of visible cytopathic effect (CPE) and it directly interacted with HRV particles. Furthermore, A549 and Hep2 cells incubated with 32 microg/mL of compound (1) for 48 h exhibited antilung and antilaryngeal cancer activities, with a viability of less than 50%.
CONCLUSIONS:
These results suggest that compound (1) may be used as a potential antiviral and anticancer agent.
J. Appl. Biol. Chem.,2013, 56(1):581-90.
Anticancer and Antimicrobial Activities of 13(E)-labd-13-ene-8α,15-diol from Brachyglottis monroi[Reference:
WebLink]
In a previous study, we reported that 13(E)-labd-13- ene-8α, 15-diol (Labd-13-ene-8,15-diol ,13E) possesses antiviral and anticancer activities.
METHODS AND RESULTS:
In this study, the anticancer and antimicrobial activities of 13(E) were evaluated against 4 cancer cell lines and 6 bacteria. 13(E) showed inhibitory effect on a variety of cancer cell lines. The IC50 values was 8.3-21.3 μg/mL. 13(E) was the most effective growth inhibitor of murine leukaemia cell lines P388, producing approximately 8.3 μg/mL of IC50 in the cytopathic effect (CPE) method. 13(E) also inhibited the growth of the gram-positive bacteria (Staphyl℃℃cus aureus, Bacillus cereus and Listeria mon℃ytogenes) and gram-negative bacteria (Vibrio parahaemolyticus, Escherichia coli and Salmonella enteritidis) with a range of minimum inhibitory concentration (MIC) values from 0.092 to 0.598 mg/mL and gram-negative bacteria were more sensitive to the compound (MIC, 0.092 mg/mL).