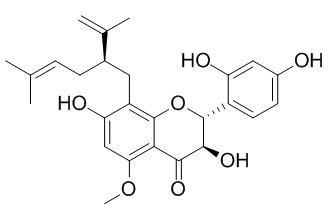
Kushenol I
Kushenol I is a GABAA receptor modulator, it exhibits inhibitory activity against Sodium-dependent glucose cotransporter 2(SGLT2).Kushenol I is shown to be active against the plant pathogenic fungus Cladosporium cucumerinum.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Res Int.2022, 157:111207.
J Phys Chem Lett.2021, 12(7):1793-1802.
BMC Complement Altern Med.2014, 14:242
Redox Rep.2024, 29(1):2392329.
Molecules.2018, 23(7):E1817
Korean J Acupunct2020, 37:104-121
Phytomedicine.2017, 24:77-86
Research Square2024, rs-4398438
Foods.2024, 13(19):3092.
Phytomedicine.2024, 155760.
Related and Featured Products
Phytochemistry, 1996, 42(5):1305-13.
Acyl secoiridoids and antifungal constituents from Gentiana macrophylla.[Pubmed:
9397205]
LC-UV-mass spectrometry and bioassay co-directed fractionation of an aqueous acetone extract of the roots of Gentiana macrophylla gave three new chromene derivatives and two novel and six known secoiridoids, along with kurarinone, Kushenol I, beta-sitosterol, stigmasterol, daucosterol, beta-sitosterol-3-O-gentiobioside, alpha-amyrin, oleanolic acid, isovitexin, gentiobiose and methyl 2-hydroxy-3-(1-beta-D-glucopyranosyl)oxybenzoate.
METHODS AND RESULTS:
The structures of the new products were established from spectral and chemical evidence as 2-methoxyanofinic acid and macrophyllosides A-D. The six known secoiridoids were gentiopicroside, sweroside, 6'-O-beta-D-glucosylgentiopicroside, 6'-O-beta-D-glucosylsweroside, trifloroside and rindoside. The new acid (2-methoxyanofinic acid), its methyl ester, kurarinone and Kushenol I were shown to be active against the plant pathogenic fungus Cladosporium cucumerinum. The methyl ester and kurarinone inhibited also the growth of the human pathogenic yeast Candida albicans. Structure-activity relationships were studied.
CONCLUSIONS:
Thus, addition of a methoxyl group to the benzene nucleus of anofinic acid (2,2-dimethyl-2H-1-benzopyran-6-carboxylic acid) increased the antifungal activity remarkably whereas glycosylation at the carboxylic moiety was found to remove the activity. Esterification of the new acid induced its activity against C. albicans, but decreased its growth inhibition properties against C. cucumerinum. Hydroxylation of kurarinone at the 3 beta-position removed its activity against C. albicans and decreased the inhibition of C. cucumerinum. In addition, the chemotaxonomic significance of the identified constituents is discussed.
Mol. Divers., 2011, 15(2):361-72.
HPLC-based activity profiling for GABAA receptor modulators from the traditional Chinese herbal drug Kushen (Sophora flavescens root).[Pubmed:
21207144 ]
METHODS AND RESULTS:
An EtOAc extract from the roots of Sophora flavescens (Kushen) potentiated γ-aminobutyric acid (GABA)-induced chloride influx in Xenopus oocytes transiently expressing GABA(A) receptors with subunit composition, α (1) β (2) γ (2S). HPLC-based activity profiling of the extract led to the identification of 8-lavandulyl flavonoids, Kushenol I, sophoraflavanone G, (-)-kurarinone, and kuraridine as GABA(A) receptor modulators. In addition, a series of inactive structurally related flavonoids were characterized. Among these, kushenol Y (4) was identified as a new natural product.
CONCLUSIONS:
The 8-lavandulyl flavonoids are first representatives of a novel scaffold for the target.