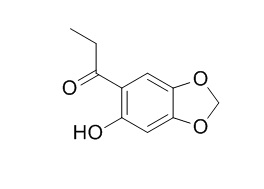
Kakuol
Kakuol has antifungal activity, it can completely inhibit the mycelial growth of Botrytis cinerea Pers ex Fr and Cladosporium cucumerinum Ellis & Arthur at 50 microg ml(-1) and 30 microg ml(-1), respectively. Kakuol and a derivative analogue are able to inhibit the DNA relaxation mediated by the human enzyme.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Genes Environ.2024, 46(1):13.
Carbohydrate Polymer Technologies & App.2021, 2:100049.
Reprod Sci.2022,10.1007/s43032-022-01117-4.
J Ethnopharmacol.2024, 335:118628.
Molecules.2021, 26(8):2161.
Front Immunol.2020, 11:598556.
Pamukkale Medical Journal2022, 15(4):796-803.
Compounds.2023, 3(1), 169-179.
Food Chem.2023, 424:136383.
Toxicol In Vitro.2019, 59:161-178
Related and Featured Products
Pest Manag. Sci., 2005, 61(8):821-5.
Isolation and antifungal activity of kakuol, a propiophenone derivative from Asarum sieboldii rhizome.[Pubmed:
15846774 ]
METHODS AND RESULTS:
An antifungal substance active against Colletotrichum orbiculare (Berk & Mont) Arx was isolated from the methanol extracts of Asarum sieboldii (Miq) Maek rhizomes. High-resolution MS, NMR and UV spectral data confirmed that the antifungal substance is Kakuol, 2-hydroxy-4,5-methylenedioxypropiophenone. Colletotrichum orbiculare was most sensitive to Kakuol, with MIC of 10 microg ml(-1). Kakuol also completely inhibited the mycelial growth of Botrytis cinerea Pers ex Fr and Cladosporium cucumerinum Ellis & Arthur at 50 microg ml(-1) and 30 microg ml(-1), respectively. However, no antimicrobial activity was found against yeast and bacteria even at 100 microg ml(-1). Kakuol exhibited a protective activity against the development of anthracnose disease on cucumber plants. The control efficacy of Kakuol against the anthracnose disease was in general somewhat less than that of the commercial fungicide chlorothalonil.
CONCLUSIONS:
This is the first report to demonstrate in vitro and in vivo antifungal activity of Kakuol against C. orbiculare infection.
Arch. Biochem. Biophys., 2013, 530(1):7-12.
A derivative of the natural compound kakuol affects DNA relaxation of topoisomerase IB inhibiting the cleavage reaction.[Pubmed:
23262316 ]
Topoisomerases IB are anticancer and antimicrobial targets whose inhibition by several natural and synthetic compounds has been documented over the last three decades.
METHODS AND RESULTS:
Here we show that Kakuol, a natural compound isolated from the rhizomes of Asarum sieboldii, and a derivative analogue are able to inhibit the DNA relaxation mediated by the human enzyme. The analogue is the most efficient one and the inhibitory effect is enhanced upon pre-incubation with the enzyme. Analysis of the different steps of the catalytic cycle indicates that the inhibition occurs at the cleavage level and does not prevent DNA binding.
CONCLUSIONS:
Molecular docking shows that the compound preferentially binds near the active site at the bottom of the catalytic residue Tyr723, providing an atomistic explanation for its inhibitory activity.
3,4-O-dimethylcedrusin
Catalog No: CFN95026
CAS No: 166021-14-3
Price: $388/10mg
Dactylorhin A
Catalog No: CFN95032
CAS No: 256459-34-4
Price: $238/20mg
Carasiphenol C
Catalog No: CFN95045
CAS No: 868168-04-1
Price: $368/5mg
Kakkalide
Catalog No: CFN95052
CAS No: 58274-56-9
Price: $260/10mg
Orcinol 1-O-beta-D-apiofuranosyl-(1->6)-beta-D-glucopyranoside
Catalog No: CFN95109
CAS No: 868557-54-4
Price: $338/5mg
[(1(10)E,2R,4R)]-2-Methoxy-8,12-epoxygemacra-1(10),7,11-trien-6-one
Catalog No: CFN95198
CAS No: 75412-95-2
Price: $318/10mg
(3R,5S,E)-1,7-Diphenylhept-1-ene-3,5-diol
Catalog No: CFN95227
CAS No: 232261-31-3
Price: $318/5mg
15-Deoxypulic acid
Catalog No: CFN95262
CAS No: 95523-05-0
Price: $368/5mg
Odontoside
Catalog No: CFN95530
CAS No: 20300-50-9
Price: $318/10mg
Yakuchinone A
Catalog No: CFN95555
CAS No: 78954-23-1
Price: $318/10mg