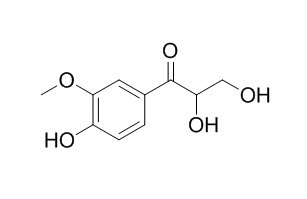
C-Veratroylglycol
C-Veratroylglycol shows antioxidant activity (IC50<100 uM).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J. Pharm. Res. Int.2022, 34(58): pp.1-14.
Horticulturae2024, 10(4), 382.
Int J Biol Sci.2023, 19(10):3077-3098.
Agronomy2023, 13(6), 1435.
Front Pharmacol.2019, 10:1226
Sci Rep.2019, 9(1):6429
Heliyon.2023, 9(12):e22932.
Evid Based Complement Alternat Med.2021, 2021:5319584.
American Association for Anatomy2020, doi: 10.1002.
Appl. Sci.2020, 10(8),2804
Related and Featured Products
Int J Mol Sci. 2017 Feb 13;18(2). pii: E392.
Hazelnut (Corylus avellana L.) Shells Extract: Phenolic Composition, Antioxidant Effect and Cytotoxic Activity on Human Cancer Cell Lines.[Pubmed:
28208804 ]
Neolignans, lawsonicin and cedrusin, a cyclic diarylheptanoid, carpinontriol B, and two phenol derivatives, C-Veratroylglycol, and β-hydroxypropiovanillone, were the main components of the extract (0.71%-2.93%, w/w).
METHODS AND RESULTS:
The biological assays suggested that the extract could be useful as a functional ingredient in food technology and pharmaceutical industry showing an in vitro scavenging activity against the radical 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) (EC50 = 31.7 μg/mL with respect to α-tocopherol EC50 = 10.1 μg/mL), and an inhibitory effect on the growth of human cancer cell lines A375, SK-Mel-28 and HeLa (IC50 = 584, 459, and 526 μg/mL, respectively). The expression of cleaved forms of caspase-3 and poly(ADP-ribose) polymerase-1 (PARP-1) suggested that the extract induced apoptosis through caspase-3 activation in both human malignant melanoma (SK-Mel-28) and human cervical cancer (HeLa) cell lines.
CONCLUSIONS:
The cytotoxic activity relies on the presence of the neolignans (balanophonin), and phenol derivatives (gallic acid), showing a pro-apoptotic effect on the tested cell lines, and the neolignan, cedrusin, with a cytotoxic effect on A375 and HeLa cells.
J Agric Food Chem. 2010 Nov 24;58(22):11673-9.
Maple syrup phytochemicals include lignans, coumarins, a stilbene, and other previously unreported antioxidant phenolic compounds.[Pubmed:
21033720 ]
Twenty-three phenolic compounds were isolated from a butanol extract of Canadian maple syrup (MS-BuOH) using chromatographic methods.
METHODS AND RESULTS:
The compounds were identified from their nuclear magnetic resonance and mass spectral data as 7 lignans [lyoniresinol (1), secoisolariciresinol (2), dehydroconiferyl alcohol (3), 5'-methoxy-dehydroconiferyl alcohol (4), erythro-guaiacylglycerol-β-O-4'-coniferyl alcohol (5), erythro-guaiacylglycerol-β-O-4'-dihydroconiferyl alcohol (6), and [3-[4-[(6-deoxy-α-l-mannopyranosyl)oxy]-3-methoxyphenyl]methyl]-5-(3,4-dimethoxyphenyl)dihydro-3-hydroxy-4-(hydroxymethyl)-2(3H)-furanone (7)], 2 coumarins [scopoletin (8) and fraxetin (9)], a stilbene [(E)-3,3'-dimethoxy-4,4'-dihydroxystilbene (10)], and 13 phenolic derivatives [2-hydroxy-3',4'-dihydroxyacetophenone (11), 1-(2,3,4-trihydroxy-5-methylphenyl)ethanone (12), 2,4,5-trihydroxyacetophenone (13), catechaldehyde (14), vanillin (15), syringaldehyde (16), gallic acid (17), trimethyl gallic acid methyl ester (18), syringic acid (19), syringenin (20), (E)-coniferol (21), C-Veratroylglycol (22), and catechol (23)]. The antioxidant activities of MS-BuOH (IC50>1000 μg/mL), pure compounds, vitamin C (IC50=58 μM), and a synthetic commercial antioxidant, butylated hydroxytoluene (IC50=2651 μM), were evaluated in the diphenylpicrylhydrazyl (DPPH) radical scavenging assay.
CONCLUSIONS:
Among the isolates, the phenolic derivatives and coumarins showed superior antioxidant activity (IC50<100 μM) compared to the lignans and stilbene (IC50>100 μM). Also, this is the first report of 16 of these 23 phenolics, that is, compounds 1, 2, 4-14, 18, 20, and 22, in maple syrup.
Zhongguo Zhong Yao Za Zhi. 2013 Dec;38(24):4329-34.
Chemical constituents of Osmanthus fragrans fruits.[Pubmed:
24791540 ]
METHODS AND RESULTS:
By Silica gel, Sephadex LH-20 and other materials for isolation and purification and by physicochemical methods and spectral analysis for structural identification, 23 compounds were isolated and identified from ethyl acetate portion of alcohol extract solution of Osmanthus fragrans fruits. Their structures were identified as nicotinamide (1), D-allitol (2), 5-hydroxymethyl-2-furancarboxaldehyde (3), acetyloleanolic acid (4), benzoic acid (5), ergosta-7,22-dien-3-one (6), beta-sitosterol (7), borreriagenin (8), cerevistero (9), C-Veratroylglycol (10), methyl-2-O-beta-glucopyranosylbenzoate (11), 3', 7-dihydroxy-4'-methoxyisoflavon (12), umbelliferone (13), caffeic acid methyl ester (14), oleanolic acid (15), (-) -chicanine (16), dillapiol (17), 3beta,5alpha, 9alpha-trihydroxyergosta-7-22-dien-6-one (18), 2alpha-hydroxy-oleanolic acid (19), betulinic acid (20), betulin (21), 3, 3'-bisdemethylpinoresinol (22), and lupeol (23).
CONCLUSIONS:
All compounds were isolated from the osmanthus fruit for the first time. Except for compounds 4, 7, 15, 19, 23, the rest ones were isolated from the this plant for the first time.