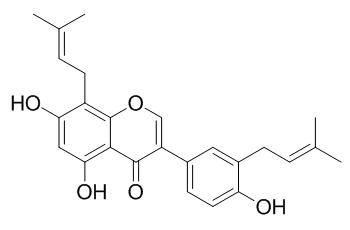
Isolupalbigenin
Isolupalbigenin shows promising cytotoxic effects toward HL-60 cells (IC50 4.3 ± 0.7 to 18.0 ± 1.7 uM), it also shows in vitro inhibitory activity toward human glyoxalase I. Isolupalbigenin shows two different antibacterial activities against MRSA: direct growth inhibition and intensification of methicillin sensitivity, it could lead to the development of compounds for new approaches against MRSA infection.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Plants (Basel).2024, 13(19):2793.
Pharmacol Rep.2017, 69(6):1224-1231
Int J Mol Sci.2018, 19(2)
Processes2022, 10(10), 2008.
Biol Pharm Bull.2018, 41(11):1685-1693
Agronomy2020, 10(3),388.
Front Microbiol.2024, 15:1429027.
Cells.2024, 13(14):1229.
Food Res Int.2020, 128:108778
Molecules.2019, 24(12):E2286
Related and Featured Products
J Asian Nat Prod Res. 2017 May;19(5):510-518.
Prenylated isoflavones from Cudrania tricuspidata inhibit NO production in RAW 264.7 macrophages and suppress HL-60 cells proliferation.[Pubmed:
27649772]
METHODS AND RESULTS:
Inhibitory effects of NO production in RAW 264.7 macrophages guided the isolation of nine prenylated isoflavones, including a new cudraisoflavone L (1) and eight known metabolites furowanin B (2), erysubin A (3), wighteone (4), lupalbigenin (5), laburnetin (6), Isolupalbigenin (7), 6,8-diprenylorobol (8), millewanin H (9) from the leaves of Cudrania tricuspidata.
CONCLUSIONS:
At the concentration of 10 μM, compounds 1, 2, and 4 significantly inhibited NO production with the inhibitory values of 72.5 ± 2.4, 66.9 ± 1.8, and 55.4 ± 2.7%, respectively. In addition, all of isolated compounds 1-9 showed promising cytotoxic effects toward HL-60 cells (IC50 4.3 ± 0.7 to 18.0 ± 1.7 μM).
Lett Appl Microbiol. 2006 Sep;43(3):243-8.
Different antibacterial actions of isoflavones isolated from Erythrina poeppigiana against methicillin-resistant Staphylococcus aureus.[Pubmed:
16910926 ]
To screen six isoflavones isolated from Erythrina poeppigiana (Leguminosae) for their antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA).
METHODS AND RESULTS:
Stem bark of E. poeppigiana was macerated with acetone and the methylene chloride-soluble fraction of the residue was applied to repeated silica gel column chromatography and eluted. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined by a broth dilution method. Inactive compounds that failed inhibiting bacterial growth at 25 microg ml(-1) were further investigated for their combination effects with methicillin and oxacillin. Of the isolated isoflavones, 5,7,4'-trihydroxy-8,3'-di(gamma,gamma-dimethylallyl)isoflavone (Isolupalbigenin) exhibited the highest anti-MRSA activity (MICs: 1.56-3.13 microg ml(-1); MBCs: 6.25-12.5 microg ml(-1)), followed by 5,7,4'-trihydroxy-6-gamma,gamma-dimethylallylisoflavone (erythrinin B). Inactive compounds were combined with methicillin or oxacillin, 5,4'-dihydroxy-(3'',4''-dihydro-3''-hydroxy)-2'',2''-dimethylpyrano[5'',6'':6,7]isoflavone (M-Wi-2) intensifying the susceptibility of MRSA strains to these antibiotics. In all but one strain, the MIC values of methicillin were reduced from > or =100 to 6.25-12.5 microg ml(-1) in the presence of M-Wi-2 (25 microg ml(-1)).
CONCLUSIONS:
Isoflavones from E. poeppigiana showed two different antibacterial activities against MRSA: direct growth inhibition and intensification of methicillin sensitivity. Isolupalbigenin and M-Wi-2 could lead to the development of compounds for new approaches against MRSA infection.
J Nat Med. 2014 Jul;68(3):636-42.
Phenolic constituents from stem bark of Erythrina poeppigiana and their inhibitory activity on human glyoxalase I.[Pubmed:
24756815 ]
METHODS AND RESULTS:
A novel isoflavone, erythgianin A (1), along with nine known compounds 2-10, was isolated from the stem bark of Erythrina poeppigiana (Leguminosae). The unusual isoflavone structure of 1, possessing a highly oxidized 3″,4″-dihydroxy-2″-hydroxymethyl-2″-methyl-2″,3″-dihydropyrano substituent, was determined on the basis of spectroscopic analyses.
CONCLUSIONS:
All of the isolated compounds were evaluated for their in vitro inhibitory activity toward human glyoxalase I. Among the isolates, Isolupalbigenin (10) with two prenyl groups showed the highest inhibitory activity.