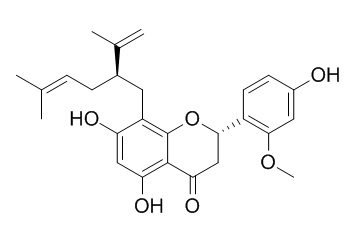
Isokurarinone
Isokurarinone is a natural product from Sophora flavescens Ait.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2024, 25(12):6456.
Chem Biodivers.2023, 20(10):e202300741.
J Pharmaceutical and Biomedical Analysis2022, 114631.
J Ethnopharmacol.2017, 198:87-90
Compounds.2023, 3(1), 169-179.
Biomed Pharmacother.2024, 174:116598.
Plant Methods.2017, 13:108
HortTechnology2016, 26(6):816-819
Phytother Res.2023, 37(10):4587-4606.
Mol Immunol. 2016, 78:121-132
Related and Featured Products
J Pharm Biomed Anal. 2007 Sep 3;44(5):1019-28.
Characterization of flavonoids in the extract of Sophora flavescens Ait. by high-performance liquid chromatography coupled with diode-array detector and electrospray ionization mass spectrometry.[Pubmed:
17658714 ]
METHODS AND RESULTS:
A method coupling high-performance liquid chromatography (HPLC) with diode-array detector (DAD) and electrospray ionization mass spectrometry (ESI) was established for the separation and characterization of flavonoids in Sophora flavescens Ait. Based on the chromatographic separation of most flavonoids present in S. flavescens Ait., a total of 24 flavonoids were identified. Fourteen compounds were unambiguously identified comparing experimental data for retention time (t(R)), UV and MS spectra with those of the authentic compounds: 3',7-dihydroxy-4'-methoxy-isoflavone (13), trifolirhizin (14), kurarinol (18), formononetin (19), 7,4'-dihydroxy-5-methoxy-8-(gamma,gamma-dimethylallyl)-flavanone (22), maackiain (21), isoxanthohumol (23), kuraridine (26), kuraridinol (27), sophoraflavanone G (30), xanthohumol (31), Isokurarinone (33), kurarinone (35) and kushenol D (38), and additional 10 compounds were tentatively identified as kushenol O (10), trifolirhizin-6''-malonate (15), sophoraisoflavanone A (20), norkurarinol/kosamol Q (24), kushenol I/N (25), kushenol C (28), 2'-methoxykurarinone (29), kosamol R (32), kushecarpin A (34) and kushenol A (37) by comparing experimental data for UV and MS spectra with those of literature. Furthermore, fragmentation pathways in positive ions mode of 24 flavonoid compounds of types of flavanone, flavanonol, flavonol, chalcone, isoflavone, isoflavanone and ptercocarpane were summarized. Some common features, such as CH(3)., H(2)O, CO, CO(2), C(3)O(2) and C(2)H(2)O losses, together with Retro-Diels-Alder fragmentations were observed in the prenylated flavonoids in S. flavescens Ait. The loss of the lanandulyl chain was their characteristic fragmentation, which might help deducing the structure of unknown flavonoid compounds.
CONCLUSIONS:
The present study provided an approach to rapidly characterize bioactive constituents in S. flavescens Ait.