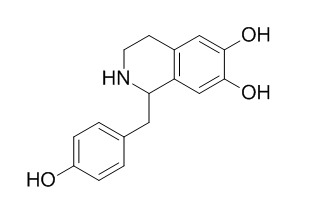
Higenamine
Higenamine (HG) is a well-known selective activator of beta2-adrenergic receptor (β2-AR) with a positive inotropic effect. HG exerts an antispasmodic effect on cold-induced vasoconstriction by regulating the PI3K/Akt, ROS/α2C-AR and PTK9 pathways independently of the AMPK/eNOS/NO axis, it reduces HMGB1 during hypoxia-induced brain injury by induction of heme oxygenase-1 through PI3K/Akt/Nrf-2 signal pathways. HG enhances the antitumor effects of cucurbitacin B in breast cancer by inhibiting the interaction of AKT and CDK2. It attenuated LPS-induced depression-like behavior by regulating BDNF-mediated ER stress and autophagy.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Chromatogr B Analyt Technol Biomed Life Sci.2020, 1149:122123.
Front Cell Dev Biol.2020, 8:32.
Pamukkale Medical Journal2022, 15(4):796-803.
Environ Toxicol.2021, doi: 10.1002
Biomolecules.2020, 10(6):925.
Evid Based Complement Alternat Med.2022, 2022:1307173.
The Pharmaceutical Society of Japan2018, 138(4):571-579
Int J Mol Sci.2024, 25(16):8846.
Cell Signal.2022, 99:110433.
Jour. of Stored Pro & Postharvest Res.2016, 7(3):32-36
Related and Featured Products
Exp Ther Med. 2019 Aug;18(2):1299-1308.
Higenamine exerts an antispasmodic effect on cold-induced vasoconstriction by regulating the PI3K/Akt, ROS/α2C-AR and PTK9 pathways independently of the AMPK/eNOS/NO axis.[Pubmed:
31316621 ]
The present study aimed to investigate the antispasmodic effect of Higenamine on cold-induced cutaneous vasoconstriction and the underlying molecular mechanisms. A cold-induced cutaneous vasoconstriction rat model was established and different doses of Higenamine were delivered by intravenous injection. The changes of cutaneous regional blood flow (RBF) between groups were analyzed.
METHODS AND RESULTS:
In vitro, the proliferation of human dermal microvascular endothelial cells was measured by MTT. The NO concentration was detected by a nitrate reductase assay. Flow cytometry was applied to measure reactive oxygen species (ROS) levels. The protein expression levels were detected by western blotting. The results demonstrated that in the model group, RBF declined compared with the normal control group, but was reversed by treatment with Higenamine. The expression of endothelial nitric oxide synthase (eNOS), phosphorylated (p)-eNOS, protein kinase B (Akt1), p-Akt1, AMP-activated protein kinase (AMPK) α1 and p-AMPKα1 was upregulated by hypothermic treatment but was reversed by Higenamine treatment. Treatment with Higenamine significantly reduced the level of intracellular α2C-adrenoreceptor (AR) compared with the hypothermia group (P<0.05). Furthermore, the expression of twinfilin-1 (PTK9) was downregulated in the Higenamine and positive control groups compared with the hypothermia group (P<0.05). Compared with the hypothermia group, the levels of ROS and α2C-AR (intracellular & membrane) were decreased in Higenamine and the positive control group (P<0.05 and P<0.01, respectively).
CONCLUSIONS:
This study, to the best of our knowledge, is the first to assess the effects of Higenamine on cold-induced vasoconstriction in vivo and its molecular mechanisms on the PI3K/Akt, AMPK/eNOS/nitric oxide, ROS/α2C-AR and PTK9 signaling pathways under hypothermia conditions. Higenamine may be a good therapeutic option for Raynaud's phenomenon (RP) and cold-induced vasoconstriction.
Biosci Rep. 2019 Jun 28;39(6).
Higenamine inhibits IL-1β-induced inflammation in human nucleus pulposus cells.[Pubmed:
31213577 ]
Intervertebral disc degeneration (IDD) is a natural progression of the aging process associated with inflammation. Higenamine, a plant-based alkaloid, has been identified to possess various pharmacological properties, including anti-inflammatory activity.
METHODS AND RESULTS:
In the present study, we aimed to evaluate the role of Higenamine in interleukin (IL)-1β-induced inflammation in human nucleus pulposus cells (NPCs). The results showed that Higenamine improved cell viability in IL-1β-induced NPCs. The IL-1β-dependent up-regulation of inflammatory molecules including inducible nitric oxide synthase (iNOS), nitric oxide (NO), prostaglandin E2 (PGE2), cyclooxygenase-2 (COX-2), tumor necrosis factor alpha (TNF-α), and IL-6 was attenuated by Higenamine in NPCs. The increased productions of matrix metalloproteinases (MMP-3 and MMP-13), as well as a disintegrin and metalloprotease with thrombospondin motifs (ADAMTS-4 and ADAMTS-5) were significantly mitigated by Higenamine treatment. Furthermore, we also found that Higenamine suppressed the IL-1β-induced activation of NF-κB signaling pathway in NPCs.
CONCLUSIONS:
In conclusion, the present study proved that Higenamine exhibited anti-inflammatory activity against IL-1β-induced inflammation in NPCs via inhibiting NF-κB signaling pathway. These results suggested that Higenamine might be a therapeutic agent for the treatment of IDD.
Apoptosis. 2012 May;17(5):463-74.
Higenamine reduces HMGB1 during hypoxia-induced brain injury by induction of heme oxygenase-1 through PI3K/Akt/Nrf-2 signal pathways.[Pubmed:
22183510]
Growing lines of evidence suggests that high mobility group box-1 (HMGB1) plays an important role for promoting inflammation and apoptosis in brain ischemia. Previously, we demonstrated that inducers of heme oxygenase-1 (HO-1) significantly reduce HMGB1 release in inflammatory conditions in vitro and in vivo. Thus, we tested our hypothesis that Higenamine protects brain injury by inhibition of middle cerebral artery occlusion (MCAO)-mediated HMGB1 release in vivo, and glucose/glucose oxidase (GOX)-induced apoptosis in C6 cells in vitro due to HO-1 induction.
METHODS AND RESULTS:
Higenamine increased HO-1 expression in C6 cells in both hypoxia and normoxia, in which the former was much more significant than the latter. Higenamine increased Nrf-2 luciferase activity, translocated Nrf-2 to nucleus, and increased phosphorylation of Akt in C6 cells. Consistent with this, LY 294002, a PI3K inhibitor, inhibited HO-1 induction by Higenamine and apoptosis induced by glucose/GOX in C6 cells was prevented by Higenamine, which effect was reversed by LY 294002. Importantly, administration of Higenamine (i.p) significantly reduced brain infarct size, mortality rate, MPO activity and tissue expression of HMGB1 in MCAO rats. In addition, recombinant high mobility group box 1 induced apoptosis in C6 cells by increasing ratio of Bax/bcl-2 and cleaved caspase c, which was inhibited by Higenamine, and all of these effects were reversed by co-treatment with ZnPPIX.
CONCLUSIONS:
Therefore, we conclude that Higenamine, at least in part, protects brain cells against hypoxic damages by up-regulation of HO-1. Thus, Higenamine may be beneficial for the use of ischemic injuries such as stroke.
Food Funct. 2019 Sep 1;10(9):6062-6073.
Natural alkaloids from lotus plumule ameliorate lipopolysaccharide-induced depression-like behavior: integrating network pharmacology and molecular mechanism evaluation.[Pubmed:
31486445 ]
Depression is a mental disorder that brings severe burdens to patients and their families. Neuroinflammation and neurotrophins are involved in depression. Lotus plumule is a nutritional food with medicinal values.
METHODS AND RESULTS:
In the present study, we tried to clarify the anti-depressive effect and molecular mechanism of lotus plumule. Network pharmacological analysis, behavior tests, qRT-PCR and western blotting were used. We found 7 potential active components and 91 targets from the TCMSP database. KEGG analysis suggested that lotus plumule significantly affected nitrogen metabolism, calcium signaling, and inflammatory mediator regulation signaling pathways. Consistent with those effects, total alkaloids of lotus plumule (TLA) and active alkaloids differently suppressed the nitric oxide (NO) production and pro-inflammatory mediators. TLA and Higenamine significantly ameliorated LPS-induced depression-like behavior, increased BDNF levels, suppressed microglia activation, and inhibited the expression of ER stress-related proteins. Meanwhile, TLA and Higenamine activated microglia autophagy by increasing the beclin-1 and LC3B-II expression. Additionally, in the presence of autophagy inhibitor 3-MA, TLA and Higenamine did not reduce the LPS-induced NO production or pro-inflammatory mediators.
CONCLUSIONS:
Collectively, TLA and Higenamine attenuated LPS-induced depression-like behavior by regulating BDNF-mediated ER stress and autophagy. Therefore, drinking tea of lotus plumule may provide a potential strategy for preventing depression.
Oncol Rep. 2018 Oct;40(4):2127-2136.
Higenamine enhances the antitumor effects of cucurbitacin B in breast cancer by inhibiting the interaction of AKT and CDK2.[Pubmed:
30106443 ]
Cucurbitacin B (Cu B), a tetracyclic triterpenoid derived from Trichosanthes kirilowii Maxim, exhibits anticancer effects against various types of tumor. Higenamine, isolated from Radix Aconiti Lateralis Preparata, has been used as a dietary supplement for regulating metabolic function.
METHODS AND RESULTS:
The present study suggested that Higenamine enhances Cu B-induced cytotoxicity in breast cancer cells and in vivo. Network pharmacology analysis was used to identify the possible mechanism of action. Cu B alone inhibited breast cancer cell growth, induced apoptosis, and arrested the cell cycle in the G2/M phase. Cu B combined with Higenamine potentiated the cytotoxic effect of Cu B, resulting in the enhanced induction of apoptosis and G2/M arrest. The network pharmacology analysis also found that the major predicted targets of Cu B in breast cancer were AKT, endoplasmic reticulum, farnesyltransferase, CAAX box, α, platelet-derived growth factor receptor α, peroxisome proliferator-activated receptor, RET proto-oncogene, and vascular endothelial growth factor A. The possible targets of Higenamine involved in the synergic action were cyclin A2, cyclin-dependent kinase 2 (CDK2), dihydrofolate reductase, and protein kinase CAMP‑activated catalytic subunit α.
CONCLUSIONS:
The associated pathways were summarized by Kyoto Encyclopedia of Genes and Genomes pathway analysis, and it was hypothesized that Higenamine may enhance the antitumor effects of Cu B in breast cancer through inhibition of the interaction of AKT and CDK2. The protein expression was assayed by western blot analysis. The combined treatment also resulted in significant inhibition of growth in vivo.
Biomed Pharmacother. 2019 Jul;115:108881.
Protective effects of higenamine combined with [6]-gingerol against doxorubicin-induced mitochondrial dysfunction and toxicity in H9c2 cells and potential mechanisms.[Pubmed:
31028997 ]
Higenamine (HG) is a well-known selective activator of beta2-adrenergic receptor (β2-AR) with a positive inotropic effect. The present study showed that HG combined with [6]-gingerol (HG/[6]-GR) protects H9c2 cells from doxorubicin (DOX)-induced mitochondrial energy metabolism disorder and respiratory dysfunction.
METHODS AND RESULTS:
H9c2 cells were pretreated with HG/[6]-GR for 2 h before DOX treatment in all procedures. Cell viability was quantified by a cell counting kit‑8 assay. Cardiomyocyte morphology, proliferation, and mitochondrial function were detected by a high content screening (HCS) assay. Cell mitochondrial stress was measured by a Seahorse XFp analyzer. To further investigate the protective mechanism of HG/[6]-GR, mRNA and protein expression levels of PPARα/PGC-1α/Sirt3 pathway-related molecules were detected. The present data demonstrated that protective effects of HG/[6]-GR combination were presented in mitochondria, which increased cell viability, ameliorated DOX-induced mitochondrial dysfunction, increased mitochondrial oxygen consumption rate (OCR) and extracellular acidification rate (ECAR). Most importantly, the protective effects were abrogated by GW6471 (a PPARα inhibitor) and ameliorated by Wy14643 (a PPARα agonist). Moreover, the combined use of HG and [6]-GR exerted more profound protective effects than either drug as a single agent.
CONCLUSIONS:
In conclusion, the results suggested that HG/[6]-GR ameliorates DOX-induced mitochondrial energy metabolism disorder and respiratory function impairment in H9c2 cells, and it indicated that the protective mechanism may be related to upregulation of the PPARα/PGC-1α/Sirt3 pathway, which promotes mitochondrial energy metabolism and protects against heart failure.