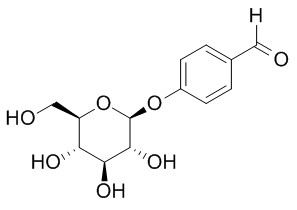
Helicid
Helicid analogues are mushroom tyrosinase inhibitors, some of them have more potent inhibitory activities than arbutin (IC50 =7.3 mM).Some helicid analogues exhibit potent cholinesterase (AChE) inhibitory activities.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Current Analytical Chemistry2024, 20(8):599-610.
Korean J. of Horticultural Sci. & Tech. 2017, 793-804
Trop J Nat Prod Res, February2023, 7(2):2371-2381
Green Chemistry2021, ISSUE 2.
Evid Based Complement Alternat Med.2021, 8855980.
Food Engineering Progress2019, 23(3)209-216
Molecules.2023, 28(13):4971.
Sustainable Chemistry & Pharmacy2022, 30:100883.
PLoS One.2020, 15(2):e0220084.
iScience.2024, 4790628.
Related and Featured Products
J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Apr 15;988:8-12.
Bioavailability and pharmacokinetics profile of helicid in beagle dogs using gradient elution high performance liquid chromatography electrospray ionization mass spectrometry.[Pubmed:
25743699]
A simple, sensitive and reliable gradient elution high performance liquid chromatography electrospray ionization mass spectrometry (HPLC-ESI-MS) method was developed for quantifying Helicid in dog plasma.
METHODS AND RESULTS:
The limit of detection (LOD) and the lower limit of quantitation (LLOQ) were 0.3 and 1 ng/mL, respectively. This method was validated for selectivity, linearity, accuracy and precision, extraction recoveries, matrix effects, carry-over, cross-talk, dilution integrity, stability and incurred sample reanalysis (ISR). Bioavailability and pharmacokinetic parameters of Helicid in beagle dogs were researched from a two period crossover design study. After intravenous administration (i.v.), Helicid had a mean (± SD) AUC0-∞ of 12062.06 ± 2482.69 ng/mL h and terminal half-life (t1/2 z) of 2.91 ± 1.37 h, while Cmax was 35613.23 ± 8157.18 ng/mL. Following intragastric gavage administration (i.g.), AUC0-∞ was 7589.16 ± 1797.20 ng/mL h along with a longer t1/2 z of 4.10 ± 4.35 h. Cmax was researched at 0.58 ± 0.20 h. The absolute bioavailability (F) of Helicid was 15.74 ± 1.87%.
Bioorg Med Chem Lett. 2008 Dec 15;18(24):6490-3.
Synthesis and biological evaluation of helicid analogues as mushroom tyrosinase inhibitors.[Pubmed:
18996693 ]
METHODS AND RESULTS:
A series of Helicid analogues were synthesized and evaluated as tyrosinase inhibitors. The results demonstrated that some compounds had more potent inhibitory activities than arbutin (IC(50) 7.3 mM). In particular, compound 1c bearing 4,6-O-benzylidene substituent on the sugar moiety was found to be the most potent inhibitor with IC(50) value of 0.052 mM. The inhibition kinetics analyzed by Lineweaver-Burk plots revealed that Helicid analogues were competitive inhibitors.
CONCLUSIONS:
The Circular dichroism spectra indicated that those compounds induced conformational changes of mushroom tyrosinase upon binding.
Eur J Med Chem. 2008 Jan;43(1):166-73.
Synthesis and biological evaluation of helicid analogues as novel acetylcholinesterase inhibitors.[Pubmed:
17574306 ]
METHODS AND RESULTS:
A series of Helicid analogues were prepared and evaluated in vitro for the cholinesterase (AChE and BuChE) inhibitory activities via UV spectroscopy. The results indicated that compounds 5, 6d and 8 exhibited potent AChE inhibitory activities with IC(50) values of 0.45+/-0.02microM, 0.49+/-0.02microM, and 0.20+/-0.01microM, respectively. High selectivity for AChE over BuChE was also observed.
CONCLUSIONS:
Kinetic study showed that the mechanism of AChE inhibition of compounds 5, 6d and 8 was all mixed-type.
Spectrochim Acta A Mol Biomol Spectrosc. 2014 Apr 24;124:46-51.
Binding of helicid to human serum albumin: a hybrid spectroscopic approach and conformational study.[Pubmed:
24463239]
METHODS AND RESULTS:
The interaction between human serum albumin and Helicid was studied by steady-state fluorescence, ultraviolet-visible, circular dichroism, Fourier transform infrared techniques and molecular modeling. The binding site numbers, association constants, and corresponding thermodynamic parameters were used to investigate the quenching mechanism. The alternations of protein secondary structure in the presence of Helicid were demonstrated using synchronous fluorescence, Fourier transform infrared, circular dichroism and three-dimensional fluorescence spectra.
CONCLUSIONS:
The molecular modeling results revealed that Helicid could bind to hydrophobic pocket of HSA with hydrophobic and hydrogen bond force. The binding site of Helicid in HSA was ascertained. Moreover, an apparent distance of 3.33 nm between the Trp214 and Helicid was obtained via fluorescence resonance energy transfer method.