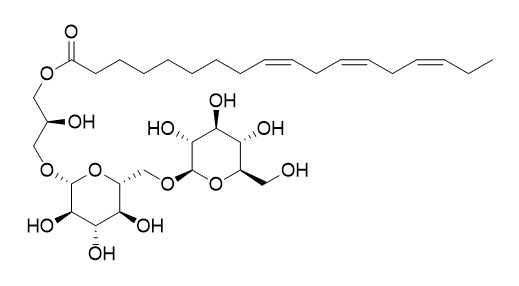
Gingerglycolipid A
Gingerglycolipid A has potent anti-ulcer activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int. Conference on Med. Sci. and Bio.2017, 17973
Phytomedicine.2024, 126:155442.
Molecules.2023, 28(19):6775.
Mol Plant Pathol.2022, 10.1111:mpp.13280.
Biochem Biophys Res Commun.2019, 518(4):732-738
Food Science and Preservation2024, 31(3):486-498.
Ulm University Medical Center2020, doi: 10.18725.
Pharmaceutics.2023, 15(9):2355.
Food and Bioprocess Technology2017, 10(6):1074-1092
HIV Med.2021, 22(8):690-704.
Related and Featured Products
Chem Pharm Bull (Tokyo) . 1994 Jun;42(6):1226-30
Stomachic principles in ginger. III. An anti-ulcer principle, 6-gingesulfonic acid, and three monoacyldigalactosylglycerols, gingerglycolipids A, B, and C, from Zingiberis Rhizoma originating in Taiwan[Pubmed:
8069973]
An anti-ulcer constituent, 6-gingesulfonic acid, and three monoacyldigalactosylglycerols, gingerglycolipids A, B, and C, were isolated from Zingiberis Rhizoma, the dried rhizome of Zingiber officinale Roscoe which was cultivated in Taiwan, together with (+)-angelicoidenol-2-O-beta-D-glucopyranoside. Based on chemical reactions and physicochemical evidence, the structures of 6-gingesulfonic acid, gingerglycolipids A, B, and C have been determined. In addition, the absolute stereostructure of (+)-angelicoidenol-2-O-beta-D-glucopyranoside was clarified on the basis of its synthesis from d-borneol. 6-Ginesulfonic acid showed weaker pungency and more potent anti-ulcer activity than 6-gingerol and 6-shogaol.
Int J Mol Sci . 2021 Apr 25;22(9):4476.
Identification of Compounds with Potential Therapeutic Uses from Sweet Pepper ( Capsicum annuum L.) Fruits and Their Modulation by Nitric Oxide (NO)[Pubmed:
33922964]
Plant species are precursors of a wide variety of secondary metabolites that, besides being useful for themselves, can also be used by humans for their consumption and economic benefit. Pepper (Capsicum annuum L.) fruit is not only a common food and spice source, it also stands out for containing high amounts of antioxidants (such as vitamins C and A), polyphenols and capsaicinoids. Particular attention has been paid to capsaicin, whose anti-inflammatory, antiproliferative and analgesic activities have been reported in the literature. Due to the potential interest in pepper metabolites for human use, in this project, we carried out an investigation to identify new bioactive compounds of this crop. To achieve this, we applied a metabolomic approach, using an HPLC (high-performance liquid chromatography) separative technique coupled to metabolite identification by high resolution mass spectrometry (HRMS). After chromatographic analysis and data processing against metabolic databases, 12 differential bioactive compounds were identified in sweet pepper fruits, including quercetin and its derivatives, L-tryptophan, phytosphingosin, FAD, Gingerglycolipid A, tetrahydropentoxylin, blumenol C glucoside, colnelenic acid and capsoside A. The abundance of these metabolites varied depending on the ripening stage of the fruits, either immature green or ripe red. We also studied the variation of these 12 metabolites upon treatment with exogenous nitric oxide (NO), a free radical gas involved in a good number of physiological processes in higher plants such as germination, growth, flowering, senescence, and fruit ripening, among others. Overall, it was found that the content of the analyzed metabolites depended on the ripening stage and on the presence of NO. The metabolic pattern followed by quercetin and its derivatives, as a consequence of the ripening stage and NO treatment, was also corroborated by transcriptomic analysis of genes involved in the synthesis of these compounds. This opens new research perspectives on the pepper fruit's bioactive compounds with nutraceutical potentiality, where biotechnological strategies can be applied for optimizing the level of these beneficial compounds.
Zhongguo Zhong Yao Za Zhi . 2008 Jan;33(1):42-6.
Studies on chemical constituents from roots of Mirabilis jalapa[Pubmed:
18338618]
Objective: To investigate the anti-HIV constituents from the root of Mirabilis jalapa.
Method: The compounds were isolated by column chromatography on silica gel, Sephadex LH - 20, MCI-gel CHP-20P and RP-18. The structure were identified by means of NMR and MS analyses (1H-NMR, 13C-NMR, MS).
Result: Eleven compounds were isolated and identified as astragaloside II (1), astragaloside II (2), astragaloside IV (3), astragaloside VI (4), flazin (5), 4'-hydroxy-2, 3-dihydroflavone 7-beta-D-glucopyranoside (6), Gingerglycolipid A (7), 3, 4-dihydroxybenzaldehyd (8), p-hydroxybenzaldehyde (9), beta-sitosterol (10) and daucosterol (11).
Conclusion: Compounds 1-9 were obtained from this genus for the first time.
Planta Med . 2012 May;78(7):703-710.
Metabolite profiling of the leaves of the Brazilian folk medicine Sideroxylon obtusifolium[Pubmed:
22322398]
Sideroxylon obtusifolium (Roem. & Schult.) T. D. Penn. (family Sapotaceae) is a tree native to Central and South America. Infusions of the bark and the leaves are used in Brazilian folk medicine as an anti-inflammatory remedy. However, information on the constituents of S. obtusifolium remains scarce, and only common pentacyclic triterpenoids have been previously reported. HPLC-DAD/MS analyses revealed that saponins and flavonoids were the main constituents of the leaves. From the butanol-soluble fraction of an ethanolic extract, a total of four saponins and ten flavonol glycosides were isolated by a combination of chromatographic methods including Sephadex LH-20, MPLC, and HPLC. Their structures were established by acid hydrolysis and spectroscopic methods, mainly MS (n), 1D and 2D NMR experiments. The compounds include the new triterpene glycoside 3-O-( β-D-glucopyranosyl)-protobassic acid 28-O- β-D-apiofuranosyl-(1 → 3)-O-[O- β-D-apiofuranosyl-(1 → 3)- β-D-xylopyranosyl-(1 → 4)]-O- α-L-rhamnopyranosyl-(1 → 2)- α-L-arabinopyranosyl ester ( 1), as well as the new flavonol glycosides, quercetin-3-O-(O- α-L-rhamnopyranosyl-(1→ 2)-O-[ β-D-glucopyranosyl-(1 → 3)]- β-D-galactopyranoside) ( 6) and kaempferol-3-O-(O- α-L-rhamnopyranosyl-(1 → 2)-O-[ β-D-glucopyranosyl-(1 → 3)]- β-D-galactopyranoside) ( 8). In addition, catechin and a glycerogalactolipid, Gingerglycolipid A, were obtained from the ethyl acetate-soluble fraction. The isolated compounds could be used in the future as chemical markers for quality control of this herbal drug.