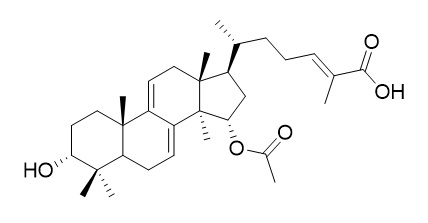
Ganoderic acid X
Ganoderic acid X is a potential Mdm2 inhibitor(K(i) = 16nM). It is a potential anticancer drug, inhibits topoisomerases and induces apoptosis of cancer cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Nutrients.2024, 16(20):3521.
Food Chem X.2024, 24:101909.
Aging (Albany NY).2021, 13(19):22867-22882.
Journal of Food Hygiene and Safety2019, 34(5):413-420
J Applied Biological Chemistry2021, 64(2):185-192
Int J Mol Sci.2023, 24(7):6360.
J Herbmed Pharmacol.2018, 7(4):280-286
World J Microbiol Biotechnol.2024, 40(9):265.
Pharmacognosy Journal, 2021, 13(5).
Molecules.2022, 27(21):7643.
Related and Featured Products
Life Sci. 2005 Jun 3;77(3):252-65.
Ganoderic acid X, a lanostanoid triterpene, inhibits topoisomerases and induces apoptosis of cancer cells.[Pubmed:
15878354 ]
Lanostanoid triterpenes isolated from Ganoderma amboinense were found to inhibit the growth of numerous cancer cell lines, and some of them inhibited the activities of topoisomerases I and IIalpha in vitro.
METHODS AND RESULTS:
Among the bioactive isolates, one of the most potent triterpene was identified to be 3 alpha-hydroxy-15 alpha-acetoxy-lanosta-7,9(11),24-trien-26-oic acid, Ganoderic acid X (GAX). Treatment of human hepatoma HuH-7 cells with GAX caused immediate inhibition of DNA synthesis as well as activation of ERK and JNK mitogen-activated protein kinases, and cell apoptosis.
CONCLUSIONS:
Molecular events of apoptosis including degradation of chromosomal DNA, decrease in the level of Bcl-xL, the disruption of mitochondrial membrane, cytosolic release of cytochrome c and activation of caspase-3 were elucidated.
The ability of GAX to inhibit topoisomerases and to sensitize the cancer cells toward apoptosis fulfills the feature of a potential anticancer drug.
J Enzyme Inhib Med Chem. 2013 Jun;28(3):569-75.
Virtual screening of low molecular weight mushrooms compounds as potential Mdm2 inhibitors.[Pubmed:
22380771]
In some human cancer cases, the activity of p53 is inhibited by over-expressed Mdm2. The Mdm2 acts as an ubiquitin ligase, resulting in p53 ubiquitination and subsequent p53 proteasomal degradation. The disruption of the Mdm2-p53 interaction using small-molecule inhibitors is recognized as a promising strategy for anti-cancer drug design. Mushrooms are an important source of powerful compounds with anti-tumour properties.
METHODS AND RESULTS:
In this study, the first virtual screening of low molecular weight compounds present in mushroom is presented as potential Mdm2 inhibitors. A re-docking and cross-docking method was used to validate the virtual screening protocol.
The steroids: Ganoderic acid X (K(i) = 16nM), ganoderic acid Y (K(i) = 22nM) and ganoderic acid F (K(i) = 69nM); 5,6-epoxy-24(R)-methylcholesta-7,22-dien-3β-ol (K(i) = 74nM) and polyporenic acid C (K(i) = 59nM) stand out as the top ranked potential inhibitors of Mdm2.
CONCLUSIONS:
The docking pose of the most promising compounds were carefully analysed and the information provided shows several interesting starting points for further development of Mdm2 inhibitors.