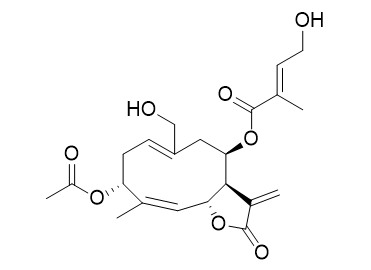
Eupalinolide H
Eupalinolide H, a sesquiterpene lactone, has the potential to be used as natural anti-inflammatory agent.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biochem Biophys Res Commun.2019, 518(4):732-738
Front Aging Neurosci.2019, 11:230
Arch Biochem Biophys.2024, 759:110111.
Bioorg Chem.2024, 152:107720.
Korean j.of Pharm.2017, 70-76
J Pharm Biomed Anal.2017, 140:274-280
J Chromatogr A.2024, 1714:464544.
Front Chem.2023, 11:1245071.
Int J Mol Sci.2022, 23(23):14826.
Molecules.2024, 29(23):5792.
Related and Featured Products
Nat Prod Res . 2019 Feb;33(4):477-485
Precise discovery of a STAT3 inhibitor from Eupatorium lindleyanum and evaluation of its activity of anti-triple-negative breast cancer[Pubmed:
29086600]
Michael reaction acceptors (MRAs) are a class of active compounds. There is a great prospect to screen STAT3 inhibitors from Eupatorium lindleyanum, furthermore, to discover lead compounds for anti-triple-negative breast cancer (TNBC). In this study, glutathione (GSH) was employed, and a UPLC-MS screening method was developed to discover MRAs. We screened MRAs which can inhibit STAT3 using a STAT3-dependent reporter system. Six sesquiterpene lactones, including a new compound Eupalinolide O (1), together with five known compounds, Eupalinolide I (2), Eupalinolide K (3), Eupalinolide H (4), Eupalinolide J (5) and Eupalinolide G (6) were isolated. Eupalinolide J was identified as MRA that decreased luciferase activity of STAT3. Preliminary activity assessment showed that Eupalinolide J could inhibit the viability of TNBC cell lines. We demonstrated that Eupalinolide J, which is a natural typical MRA, has a notable inhibition of STAT3 activity and a potential cytotoxic activity against TNBC cell lines.
J Asian Nat Prod Res . Apr-Aug 2007;9(3-5):339-45.
Cytotoxic sesquiterpene lactones from Eupatorium lindleyanum[Pubmed:
17613619]
Three new germacrane sesquiterpenes, eupalinolides C-E (1-3), along with three known germacrane sesquiterpenes, eupalinolide A (4), eupalinolide B (5), and 3beta-acetoxy-8beta-(4'-hydroxytigloyloxy)-14-hydroxycostunolide (6), were isolated from Eupatorium lindleyanum. They were tested for cytotoxicity against A-549, BGC-823, SMMC-7721, and HL-60 tumour cell lines. The results showed that these compounds demonstrated potent cytotoxicity. The structures of the compounds were elucidated by means of (1)H and (13)C NMR spectroscopic analysis, including 2D NMR experiments.
Planta Med . 2018 Jan;84(2):123-128.
Potential Anti-inflammatory Sesquiterpene Lactones from Eupatorium lindleyanum[Pubmed:
28793356]
Eupatorium lindleyanum has traditionally been used as folk medicine in Asian countries for its therapeutic effects on tracheitis and tonsillitis. Investigation of the anti-inflammatory active constituents from E. lindleyanum led to the isolation of two novel sesquiterpene lactones, named eupalinolide L (1: ) and eupalinolide M (2: ), and seven known sesquiterpene lactones (3: -9: ). The structures and configurations of the new compounds were determined on the basis of spectroscopic analysis, especially 2D NMR techniques. In vivo experiments showed that the sesquiterpenes fraction significantly reduced mouse ear edema induced by xylene (18.6%, p < 0.05). In in vitro assays, compounds 1: -9: showed excellent anti-inflammatory activities, as they lowered TNF-α and IL-6 levels in lipopolysaccharide-stimulated murine macrophage RAW 264.7 cells (p < 0.001). The above results suggest that the sesquiterpene lactones from E. lindleyanum can be developed as novel potential natural anti-inflammatory agents.