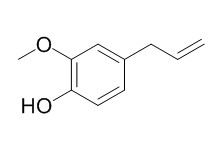
Eugenol
Eugenol is an essential oil found in cloves with herbicide,sedative,analgesic,antibacterial,anthelmintic,anti-inflammatory, cancer chemopreventive and antioxidant activities. Eugenol is shown to inhibit lipid peroxidation, the mRNA expression of COX-2, but not COX-1 and inhibit the GABAA current in trigeminal ganglion neurons. It could be developed as therapeutic agent against diseases with excessive osteoclast activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytomedicine.2022, 96:153877.
Antioxidants (Basel).2023, 12(12):2078.
Metabolites.2023, 13(5):625.
Antioxidants (Basel).2021, 10(3):379.
Pharmaceuticals (Basel).2024, 17(8):1001.
Processes 2021, 9(5),894.
ACS Omega.2021, 6(36):23460-23474.
Korean Journal of Pharmacognosy.2020, 51(2):100-106
Phytochemistry Letters2021, 43:80-87.
J Ethnopharmacol.2024, 326:117902.
Related and Featured Products
PLoS One. 2015 Jan 30;10(1):e0117316.
Eugenol inhibits the GABAA current in trigeminal ganglion neurons.[Pubmed:
25635877]
Eugenol has sedative, antioxidant, anti-inflammatory, and analgesic effects, but also serves as an irritant through the regulation of a different set of ion channels. Activation of gamma aminobutyric acid (GABA) receptors on sensory neurons leads to the stabilization of neuronal excitability but contributes to formalin-induced inflammatory pain.
METHODS AND RESULTS:
In this study, we examined the effect of Eugenol on the GABA-induced current in rat trigeminal ganglia (TG) neurons and in human embryonic kidney (HEK) 293 cells expressing the GABAA receptor α1β2γ2 subtype using the whole-cell patch clamp technique. RT-PCR and Western blot analysis were used to confirm the expression of GABAA receptor γ2 subunit mRNA and protein in the TG and hippocampus. Eugenol decreased the amplitude ratio of the GABA-induced current to 27.5 ± 3.2% (p < 0.05) in TG neurons, which recovered after a 3-min washout. In HEK 293 cells expressing the α1β2γ2 subtype, Eugenol inhibited GABA-induced currents in a dose-dependent manner. Application of Eugenol also decreased the GABA response in the presence of a G-protein blocker. Eugenol pretreatment with different concentrations of GABA resulted in similar inhibition of the GABA-induced current in a non-competitive manner. In conclusion, Eugenol inhibits the GABA-induced current in TG neurons and HEK 293 cells expressing the GABAA receptor in a reversible, dose-dependent, and non-competitive manner, but not via the G-protein pathway.
CONCLUSIONS:
We suggest that the GABAA receptor could be a molecular target for Eugenol in the modulation of nociceptive information.
Pestic Biochem Physiol. 2015 Feb;118:64-70.
Eugenol-inhibited root growth in Avena fatua involves ROS-mediated oxidative damage.[Pubmed:
25752432]
Plant essential oils and their constituent monoterpenes are widely known plant growth retardants but their mechanism of action is not well understood. We explored the mechanism of phytotoxicity of Eugenol, a monoterpenoid alcohol, proposed as a natural herbicide.
METHODS AND RESULTS:
Eugenol (100-1000 μM) retarded the germination of Avena fatua and strongly inhibited its root growth compared to the coleoptile growth. We further investigated the underlying physiological and biochemical alterations leading to the root growth inhibition. Eugenol induced the generation of reactive oxygen species (ROS) leading to oxidative stress and membrane damage in the root tissue. ROS generation measured in terms of hydrogen peroxide, superoxide anion and hydroxyl radical content increased significantly in the range of 24 to 144, 21 to 91, 46 to 173% over the control at 100 to 1000 μM Eugenol, respectively. The disruption in membrane integrity was indicated by 25 to 125% increase in malondialdehyde (lipid peroxidation byproduct), and decreased conjugated diene content (~10 to 41%). The electrolyte leakage suggesting membrane damage increased both under light as well as dark conditions measured over a period from 0 to 30 h. In defense to the oxidative damage due to Eugenol, a significant upregulation in the ROS-scavenging antioxidant enzyme machinery was observed. The activities of superoxide dismutases, catalases, ascorbate peroxidases, guaiacol peroxidases and glutathione reductases were elevated by ~1.5 to 2.8, 2 to 4.3, 1.9 to 5.0, 1.4 to 3.9, 2.5 to 5.5 times, respectively, in response to 100 to 1000 μM Eugenol.
CONCLUSIONS:
The study concludes that Eugenol inhibits early root growth through ROS-mediated oxidative damage, despite an activation of the antioxidant enzyme machinery.
Life Sci. 2003 Jun 6;73(3):337-48.
Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells.[Pubmed:
12757841]
Inducible cyclooxygenase (COX-2) has been implicated in the processes of inflammation and carcinogenesis. Thus, the potential COX-2 inhibitors have been considered as anti-inflammatory or cancer chemopreventive agents.
METHODS AND RESULTS:
In this study, the methanolic extract of the cortex of Eugenia caryophyllata Thunberg (Myrtaceae) was found to potently inhibit the prostaglandin E(2) production in lipopolysaccharide (LPS)-activated mouse macrophage RAW264.7 cells (98.3% inhibition at the test concentration of 10 microg/ml). Further, hexane-soluble layer was the most active partition compared to ethyl acetate, n-butanol, and water-soluble parts. By bioassay-guided fractionation of hexane-soluble partition, Eugenol was isolated and exhibited a significant inhibition of PGE(2) production (IC(50) = 0.37 microM). In addition, Eugenol suppressed the cyclooxygenase-2 (COX-2) gene expression in LPS-stimulated mouse macrophage cells. On the line of COX-2 playing an important role in colon carcinogenesis further study was designed to investigate the effect of Eugenol on the growth and COX-2 expression in HT-29 human colon cancer cells. Eugenol inhibited the proliferation of HT-29 cells and the mRNA expression of COX-2, but not COX-1.
CONCLUSIONS:
This result suggests that Eugenol might be a plausible lead candidate for further developing the COX-2 inhibitor as an anti-inflammatory or cancer chemopreventive agent.
J Ethnopharmacol. 2010 Jul 6;130(1):107-15.
Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane.[Pubmed:
20435121 ]
To evaluate the antibacterial activity of Eugenol and its mechanism of bactericidal action against Salmonella typhi.
METHODS AND RESULTS:
The antibacterial activity was checked by disc-diffusion method, MIC, MBC, time course assay and pH sensitivity assay. The chemo-attractant property of Eugenol was verified by chemotaxis assay. The mode of action of Eugenol was determined by crystal violet assay, measurement of release of 260 nm absorbing material, SDS-PAGE, FT-IR spectroscopy, AFM and SEM.
Treatment with Eugenol at their MIC (0.0125%) and MBC (0.025%) reduced the viability and resulted in complete inhibition of the organism. Eugenol inactivated Salmonella typhi within 60 min exposure. The chemo-attractant property of Eugenol combined with the observed high antibacterial activity at alkaline pH favors the fact that the compound can work more efficiently when given in vivo. Eugenol increased the permeability of the membrane, as evidenced by crystal violet assay. The measurement of release of 260 nm absorbing intracellular materials, SDS-PAGE, SEM and AFM analysis confirmed the disruptive action of Eugenol on cytoplasmic membrane. The deformation of macromolecules in the membrane, upon treatment with Eugenol was verified by FT-IR spectroscopy.
CONCLUSIONS:
The results suggest that the antibacterial activity of Eugenol against Salmonella typhi is due to the interaction of Eugenol on bacterial cell membrane.
Connect Tissue Res. 2014 Dec 11:1-9.
Inhibitory effects of eugenol on RANKL-induced osteoclast formation via attenuation of NF-κB and MAPK pathways.[Pubmed:
25405641]
Bone loss diseases are often associated with increased receptor activator of NF-κB ligand (RANKL)-induced osteoclast formation. Compounds that can attenuate RANKL-mediated osteoclast formation are of great biomedical interest. Eugenol, a phenolic constituent of clove oil possesses medicinal properties; however, its anti-osteoclastogenic potential is unexplored hitherto.
METHODS AND RESULTS:
Here, we found that Eugenol dose-dependently inhibited the RANKL-induced multinucleated osteoclast formation and TRAP activity in RAW264.7 macrophages. The underlying molecular mechanisms included the attenuation of RANKL-mediated degradation of IκBα and subsequent activation of NF-κB pathway. Furthermore, increase in phosphorylation and activation of RANKL-induced mitogen-activated protein kinase pathways (MAPK) was perturbed by Eugenol. RANKL-induced expression of osteoclast-specific marker genes such as TRAP, cathepsin K (CtsK) and matrix metalloproteinase-9 (MMP-9) was remarkably downregulated by Eugenol.
CONCLUSIONS:
These findings provide the first line of evidence that Eugenol mediated attenuation of RANKL-induced NF-κB and MAPK pathways could synergistically contribute to the inhibition of osteoclast formation. Eugenol could be developed as therapeutic agent against diseases with excessive osteoclast activity.
BMC Cancer. 2013 Dec 13;13:600.
Eugenol triggers apoptosis in breast cancer cells through E2F1/survivin down-regulation.[Pubmed:
24330704]
Cell lines:Breast cancer cells(MDA-MB-231, MCF7 and T47-D) and the non-tumorigenic MCF 10A cell line
Concentrations: 1, 2, 4, 10 μM
Incubation Time: 24 h
Method:
Cells are seeded into 96-well plates and incubated overnight. The medium is replaced with fresh one containing the desired concentrations of Eugenol. After 20 hrs, 10 μl of the WST-1 reagent is added to each well and the plates are incubated for 4 hrs at 37°C. The amount of formazan is quantified using ELISA reader at 450 nm of absorbance.
Free Radic Res. 1995 Dec;23(6):617-27.
The protective effects of eugenol on carbon tetrachloride induced hepatotoxicity in rats.[Pubmed:
8574354]
Animal Models: Wistar/NIN rats
Formulation: 5% soluble starch
Dosages:5 or 25 mg/kg body
Administration: p.o.
(R)-Reticuline
Catalog No: CFN95013
CAS No: 3968-19-2
Price: Inquiry(manager@chemfaces.com)
3,3',4',5,6,7,8-heptamethoxyflavone
Catalog No: CFN95021
CAS No: 1178-24-1
Price: $268/10mg
Arjunglucoside I
Catalog No: CFN95049
CAS No: 62319-70-4
Price: $268/10mg
(1E)-3-methoxy-8,12-epoxygermacra-1,7,10,11-tetraen-6-one
Catalog No: CFN95219
CAS No: 1393342-06-7
Price: $413/5mg
Cannabisin A
Catalog No: CFN95287
CAS No: 130508-46-2
Price: $318/5mg
Parishin G
Catalog No: CFN95324
CAS No: 952283-93-1
Price: $318/10mg
Melitidin
Catalog No: CFN95419
CAS No: 1162664-58-5
Price: $318/10mg
10-Methoxygambogenic acid
Catalog No: CFN95450
CAS No: 2095102-72-8
Price: $318/10mg
Herbacetin 3-sophoroside-8-glucoside
Catalog No: CFN95529
CAS No: 77298-68-1
Price: $318/5mg
Ganoderic acid GS-1
Catalog No: CFN95571
CAS No: 1206781-64-7
Price: $413/5mg