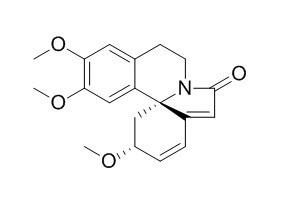
Erysotramidine
(+)-Erysotramidine shows potent dosedependant antifeedant activity at concentrations 3100 ppm.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Journal of Oil Palm Research2019, 31(2):238-247
Sci. Rep.2015, 14-23
Molecules.2024, 29(5):1050.
Food Chem.2024, 456:140044.
Eur J Pharm Sci.2016, 94:33-45
Mol Med Rep.2014, 9(5):1653-9
ACS Synth Biol.2022, doi: 10.1021.
J Cell Mol Med.2022, 26(23):5807-5819.
Bioorg Chem.2024, 152:107720.
University of Burgos2024, ssrn.4795441.
Related and Featured Products
Records of Natural Products, 2009 ,3 (2) :96-103.
Antifeedant activities of the erythrinaline alkaloids from Erythrina latissima against Spodoptera littoralis (Lepidoptera noctuidae).[Reference:
WebLink]
METHODS AND RESULTS:
The antifeedant activities of the Erythrina alkaloids from the seeds, seed pods and flowers ofErythrina latissima were investigated in laboratory dual- choice bioassays using third-instar Spodoptera littoralis(Boisduval) larvae. The new compound (+)-11-methoxy-10-oxoErysotramidine (1) from the flowers, showedpotent dose dependant activity at concentration 3 500 ppm while (+)-10,11-dioxoErysotramidine (2) also newfrom the flowers showed potent dose dependant activity at concentration 3100 ppm. Three known compounds(+)-erysotrine, (+)-Erysotramidine, (+)-erythraline, (+)-11-hydroxyErysotramidine showed potent dosedependant antifeedant activity at concentrations 3100 ppm while (+)-10,11-dioxoerysotrine and (+)-11b-hydroxyErysotramidine also a known compounds showed potent dose dependant antifeedant activity atconcentrations 3 300 ppm.
CONCLUSIONS:
Three known compounds (+)-11-methoxyErysotramidine, (+)-8-oxoerythralineand (+)-15(16)b-D-glucoerysodine showed no appreciable change in antifeedant activity with concentrationchange.
J Org Chem. 2014 Sep 5;79(17):8481-5.
Synthesis of the erythrina alkaloid erysotramidine.[Pubmed:
25140810]
METHODS AND RESULTS:
A concise synthesis of Erysotramidine (an alkaloid belonging to the erythrina family) was achieved starting with an inexpensive phenol and amine derivative. The synthesis is based on oxidative phenol dearomatizations mediated by a hypervalent iodine reagent and includes a novel route to a key indolinone moiety.
J Org Chem. 2015 Feb 6;80(3):1957-63.
Heck cyclization strategy for preparation of erythrinan alkaloids: asymmetric synthesis of unnatural (-)-erysotramidine from L-tartaric acid.[Pubmed:
25569446]
METHODS AND RESULTS:
With an imide derived from L-tartaric acid as the starting material, ent-Erysotramidine was synthesized for the first time. The synthesis features the use of the enantiopure synthon, prepared in a set of highly stereoselective reactions, including N-acyliminium cyclization, dihydrofuranyl ring formation via silver-catalyzed intramolecular alcohol addition to acetylene, and vinyl ether catalytic hydrogen reduction.
CONCLUSIONS:
The crucial step of the synthesis, assembly of ring A, was achieved by using Heck cyclization of (Z)-iodoolefin.
Org Lett. 2009 Nov 19;11(22):5230-3.
Efficient formal total synthesis of the erythrina alkaloid (+)-erysotramidine, using a domino process.[Pubmed:
19860384]
METHODS AND RESULTS:
A domino process consisting of an amidation, spirocyclization, and formation of an iminium ion and electrophilic aromatic substitution of a phenylethylamine and a ketoester leads to the spirocyclic skeleton of (+)-Erysotramidine, which can be further transformed into the natural alkaloid.