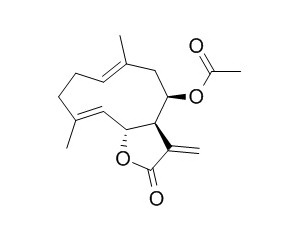
Epitulipinolide
Epitulipinolide has antitumor activity. Epitulipinolide diepoxide has antioxidative activity and chemopreventive activity in skin melanoma cells, it can significantly inhibit the proliferation of melanoma cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Fitoterapia.2022, 157:105130.
Molecules.2024, 29(22):5260.
Comput Biol Chem.2019, 83:107096
bioRxiv-Pharm.&Toxi.2022, 2022.481203.
Chemistry of Natural Compounds2020, 56,423-426
Neuropharmacology2019, 151437
Nutrients.2024, 16(19):3266.
Molecules.2019, 24(21):E3834
J Appl Microbiol.2022, 132(2):949-963.
Nat Prod Commun.2018, 10.1177
Related and Featured Products
Molecules. 2014 Apr 3;19(4):4234-45.
Antioxidant and anticancer constituents from the leaves of Liriodendron tulipifera.[Pubmed:
24705566]
METHODS AND RESULTS:
Sixteen compounds were extracted and purified from the leaves of Liriodendron tulipifera. These compounds include aporphines, oxoaporphine, coumarin, sesquiterpene lactone, benzenoids, cyclitol and steroids. (+)-Norstephalagine (2) (an aporphine) and scopoletin (8) (a coumarin) were isolated from Liriodendron tulipifera leaves from the first time. The identified compounds were screened for their antiradical scavenging, metal chelating and ferric reducing power activities. The results have showed that these compounds have antioxidative activity. The study has also examined the chemopreventive property of the isolated compounds against human melanoma cells A375. The results shown that (-)-anonaine (1), (-)-liridinine (3), (+)-lirinidine (6), lysicamine (7) and Epitulipinolide diepoxide (9) significantly inhibited the proliferation of melanoma cells.
CONCLUSIONS:
These results revealed that these compounds have antioxidative activity and chemopreventive activity in skin melanoma cells.
J. Organic Chem.,1972,37(17):2740-4.
Antitumor agents. VI. Sesquiterpene lactones tulipinolide and epitulipinolide from Liriodendron tulipifera.[Reference:
WebLink]
The dihydro derivatives 3 and 4 of tulipinolide (1) and Epitulipinolide (2) were prepared by NaBH4 reduction since catalytic hydrogenation gave other reduction products.
METHODS AND RESULTS:
The stereochemistry at the reduced center of these derivatives was determined by cyclization to the already known β-cyclo compounds 6 and 7. Minor alkaline-hydrolysis products of Epitulipinolide (2) were established as the methoxy Michael adducts 13 and 15 if methanol was a cosolvent. Without methanol the eudasmanolide diol 16 was formed along with the unusual cadinene lactone 22, but in no case was the C-8 cis γ-lactone germacranolide (isoeupatolide) detected.
CONCLUSIONS:
A product of alkaline hydrolysis of tulipinolide (1) was desacetylisotulipinolide (23), a C-8 trans γ-lactone which was also obtained by treatment of eupatolide methanesulfonate (25) with hydroxide ion. The acetate of 23 (isotulipinolide) was shown to be identical with the recently isolated germacranolide, laurenobiolide.