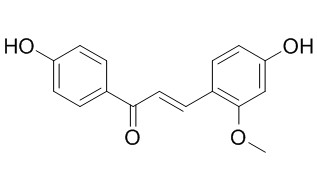
Echinatin
Echinatin has significant antioxidant and anti-inflammary activities, it shows strong scavenging activity toward the ABTS + radical, it also inhibits the production of nitric oxide (NO), interleukin-6 (IL-6) and prostaglandin E2 (PGE2) in LPS-induced macrophage cells. Echinatin disturbs the mitochondrial energy transfer reactions and membrane permeability, at a low concentration cause deterioration of respiratory control and oxidative phosphorylation of isolated rat liver mitochondria, inhibits DNP-ATPase activity while stimulating range latent ATPase activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Korean Herb. Med. Inf.2021, 9(2):231-239.
ACS Omega.2024, 9(12):14356-14367.
Molecules.2019, 24(19):E3417
J Agric Food Chem.2021, 69(46):14037-14047.
Journal of Food and Drug Analysis2023, 31(3), 9.
Food Addit Contam Part A Chem Anal Control Expo Risk Assess.2020, 37(9):1437-1448.
Am J Chin Med.2023, 51(4):1019-1039.
Journal of Functional Foods2022, 96: 105216.
Appl. Sci.2021, 11(19),9343.
J of Food Quality2020, 8851285.
Related and Featured Products
J Toxicol Sci. 1982 Nov;7(4):245-54.
The effects of echinatin and its related compounds on the mitochondrial energy transfer reaction.[Pubmed:
6221118]
METHODS AND RESULTS:
To investigate the mechanism by which various biological action of licorice root are brought about, the effects of Echinatin as a small constituent of Glycyrrhiza echinata and several related compounds on mitochondrial energy transfer reactions were examined. The results obtained were as follows: 1) Echinatin, 4'-hydroxychalcone, chalcone and 3,4'-dihydroxychalcone at a low concentration cause deterioration of respiratory control and oxidative phosphorylation of isolated rat liver mitochondria. 2) Chalcone and 4'-hydroxychalcone stimulate both latent and DNP-ATPase activity of mitochondria. Echinatin inhibits DNP-ATPase activity while stimulating range latent ATPase activity in the low concentration. 3) Chalcone and 4'-hydroxychalcone induce a rapid potassium release from mitochondrial vesicles, while Echinatin and 3,4'-dihydroxychalcone have lesser effect than the former two substances.
CONCLUSIONS:
From these results, it can be concluded that Echinatin and several related compounds disturb the mitochondrial energy transfer reactions and membrane permeability.
Food Chem. 2013 Nov 15;141(2):1063-71.
Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice.[Pubmed:
23790887]
Licorice, the roots and rhizomes of several Glycyrrhiza species (Leguminosae), is an important natural sweetening agent and a widely used herbal medicine.
METHODS AND RESULTS:
In this work, six flavonoids, 5-(1,1-dimethylallyl)-3,4,4'-trihydroxy-2-methoxychalcone (1), licochalcone B (2), licochalcone A (3), Echinatin (4), glycycoumarin (5) and glyurallin B (6), were isolated from the extracts of licorice (Glycyrrhiza inflata and Glycyrrhiza uralensis). Their structures were elucidated using various spectroscopic methods. To our knowledge, compound 1 was isolated from natural plants for the first time. All the isolates were tested by antioxidant and anti-inflammatory assays. Compounds 2, 4 and 5 showed strong scavenging activity toward the ABTS(+) radical, and compounds 1, 2, 3, 5 and 6 exhibited potent inhibition of lipid peroxidation in rat liver microsomes compared with the reference controls. Compounds 1-4 dose-dependently inhibited LPS induced reactive oxygen species (ROS) production in RAW 264.7 cells. Furthermore, compounds 1-5 were demonstrated to inhibit the production of nitric oxide (NO), interleukin-6 (IL-6) and prostaglandin E2 (PGE2) in LPS-induced macrophage cells. Moreover, the contents of the six compounds, in different Glycyrrhiza species, were quantified by HPLC-MS.
Chinese Journal of New Drugs, 2013, 22(21):2547-52.
Determination of antioxidant activity in licorice vitro metabolites by DPPH spiking coupled with HPLC-Q-TOF MS/MS[Reference:
WebLink]
To investigate the antioxidant activity of liver microsomal metabolites from six flavonoids in licorice by 1, 1-diphenyl-2-picrylhydrazyl (DPPH) spiking coupled with HPLC-Q-TOF MS/MS.
METHODS AND RESULTS:
The six flavonoids were incubated with rat liver microsomes and the resultant metabolites were identified by HPLC-Q-TOF MS/MS. The antioxidant activity of the metabolites was calculated by comparing the peak area changes before and after incubation with DPPH. The peak area of the metabolites significantly decreased or even disappeared after incubation with DPPH. Besides the metabolites Lico A-M1 and Lico A-M2 of licochalcone A, more than 90% of other metabolites of flavonoids were oxidized.
CONCLUSIONS:
Liver microsomal metabolites of Echinatin, licochalcone B, licochalcone A, 5-(1, 1-dimethylally)-3, 4, 4'-trihydroxy-2-methoxychalcone, glycycourmarin and glyurallin B still have significant antioxidant activities.
Molecules . 2018 Dec 20;24(1):3.
Antioxidant Mechanisms of Echinatin and Licochalcone A[Pubmed:
30577443]
Abstract
Echinatin and its 1,1-dimethyl-2-propenyl derivative licochalcone A are two chalcones found in the Chinese herbal medicine Gancao. First, their antioxidant mechanisms were investigated using four sets of colorimetric measurements in this study. Three sets were performed in aqueous solution, namely Cu2+-reduction, Fe3+-reduction, and 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radical (PTIO•)-scavenging measurements, while 1,1-diphenyl-2-picrylhydrazyl radical (DPPH•)-scavenging colorimetric measurements were conducted in methanol solution. The four sets of measurements showed that the radical-scavenging (or metal-reduction) percentages for both Echinatin and licochalcone A increased dose-dependently. However, Echinatin always gave higher IC50 values than licochalcone A. Further, each product of the reactions of the chalcones with DPPH• was determined using electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UPLC-ESI-Q-TOF-MS/MS). The UPLC-ESI-Q-TOF-MS/MS determination for Echinatin yielded several Echinatin⁻DPPH adduct peaks (m/z 662, 226, and 196) and dimeric Echinatin peaks (m/z 538, 417, and 297). Similarly, that for licochalcone A yielded licochalcone A-DPPH adduct peaks (m/z 730, 226, and 196) and dimeric licochalcone A peaks (m/z 674 and 553). Finally, the above experimental data were analyzed using mass spectrometry data analysis techniques, resonance theory, and ionization constant calculations. It was concluded that, (i) in aqueous solution, both Echinatin and licochalcone A may undergo an electron transfer (ET) and a proton transfer (PT) to cause the antioxidant action. In addition, (ii) in alcoholic solution, hydrogen atom transfer (HAT) antioxidant mechanisms may also occur for both. HAT may preferably occur at the 4-OH, rather than the 4'-OH. Accordingly, the oxygen at the 4-position participates in radical adduct formation (RAF). Lastly, (iii) the 1,1-dimethyl-2-propenyl substituent improves the antioxidant action in both aqueous and alcoholic solutions.
Keywords: 1,1-dimethyl-2-propenyl; antioxidant; dimer; Echinatin; licochalcone A; radical adduct formation; α,α-dimethyl-β-propenyl.