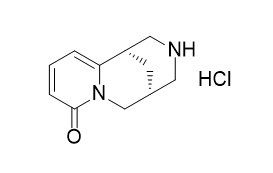
Cytisine Hydrochloride
Cytisine hydrochloride has toxicity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Dental Journal2024, 57(4): 254-258
J Ethnopharmacol.2023, 309:116302.
CZECH MYCOLOGY2021, 73(1):1-19.
Molecules.2019, 24(9):E1719
Int J Mol Sci.2021, 22(10):5181.
Evid Based Complement Alternat Med.2018, 2018:4259603
Kaohsiung J Med Sci.2023, 10.1002/kjm2.12764
Front Pharmacol.2021, 12:765521.
Jeju National University Graduate School2023, 24478
Separations2021, 8(1), 1.
Related and Featured Products
Br J Pharmacol. 1969 Jan;35(1):161-74.
Some studies on cytisine and its methylated derivatives.[Pubmed:
4387392]
METHODS AND RESULTS:
1. In mice Cytisine Hydrochloride is less toxic intravenously than nicotine hydrogen tartrate, but more toxic by intraperitoneal or oral administration.
Compared with cytisine, caulophylline hydrogen iodide is one-fifth to one-tenth as toxic and caulophylline methiodide is less than one-thirtieth as toxic.2. The surprising low oral toxicity of cytisine and nicotine may be ascribed to the method of administration; if the drug is placed directly in the stomach there is no possibility of absorption through buccal mucous membranes.3. The peripheral effects of nicotine, cytisine and caulophylline are similar, though on some preparations those of nicotine last longer. In most tests cytisine is active in doses from a quarter to three-quarters of those of nicotine, caulophylline in doses from 10 to 20 times those of cytisine. Caulophylline methiodide is virtually inactive.4. Cytisine and caulophylline may differ from nicotine in their central effects.5. Cytisine and caulophylline are active as the cations. The pKa of cytisine is 7.92 and that of caulophylline is 7.04; the difference accounts, in part, for the weaker activity of caulophylline. The caulophylline ion is generally one-sixth to one-third as active as the cytisine ion.6. The introduction of the second methyl group to form the quaternary salt does not appear to cause a dramatic change in the conformation of the molecule.
CONCLUSIONS:
Caulophylline methiodide appears to be feebly active because it has feeble affinity.