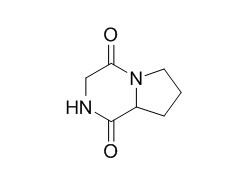
Cyclo(Pro-Gly)
Cyclo(Pro-Gly) is an active metabolite of piracetam-N-phenylacetyl-L-prolylglycine (GWS-111), it shows a greater resistance to an enzymatic effect than natural neuropeptides. Cyclo-(Gly-Pro) shows cytotoxicity at the concentration of 10 umol/L, it inhibits the growth of Bacillus subtilis with the minimal inhibitory concentration (MIC) value of 0.8, 0.8 g/L.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Journal of Functional Foods2022, 91:105019.
BMC Complement Altern Med.2018, 18(1):303
Mol Neurobiol.2022, 02873-9.
Neuroscience.2024, 559:77-90.
Aging (Albany NY).2023, 15(24):15557-15577.
Fitoterapia.2022, 157:105130.
Phytomedicine.2022, 100:154085.
University of East Anglia2023, 93969.
Int J Mol Sci.2024, 25(2):764.
J Ethnopharmacol.2017, 206:327-336
Related and Featured Products
Microbiology China,2010, 37(10):1475-80.
The Endophytic Fungus Strain FTJZZJ09 Isolated from the Bulbs of Fritillaria thunbergii and Its Antibacterial Metabolites[Reference:
WebLink]
METHODS AND RESULTS:
In the present study, an endophytic fungus isolate FTJZZJ09, which isolated from the fresh bulbs of Fritillaria thunbergii Miq., was identified as Penicillium chrysogenum based on its morphological characters and internal transcribed spacer (ITS) sequence. After being cultured in the modified CzapeK-DoX medium (3 g/L maltose, 3 g/L peptone A, 0.1 g/L K2HPO4, 0.05 g/L KCl, 0.3 g/L NaNO3, 0.05 g/L MgSO4·7H2O, 0.001 g/L FeSO4·7H2O, pH 6.5), it can secrete antibacterial metabolites under the condition of 28°C in a rotary shaker at 160 r/min for 7 days. Three antibacterial compounds were isolated from the ethyl acetate extract of the fermentation broth by silica gel, they were elucidated as Cyclo(Pro-Gly) , cyclo (Pro-Val) and 2-acetyl-4 (3H) quinazolinone.
CONCLUSIONS:
All the three compounds could inhibit the growth of Bacillus subtilis with the minimal inhibitory concentration (MIC) value of 0.8, 0.8, and 0.4 g/L respectively, while they showed no apparent effects against the growth of Gram-negative bacteria.
Chinese Journal of Antibiotics, 2006, 31(1):36-38,62.
The antitumor active component from marine derived actinomycete S1001[Reference:
WebLink]
METHODS AND RESULTS:
To explore the antitumor active component in fermentation of a marine derived actinomycete S1001,8 compounds were isolated and purified by using solvent extraction,silica gel column and preparative HPLC.The separation procedure was traced by a bioassay using tsFT210 cell lines.By means of physico-chemical properties and spectral analyses,the structures of compounds were determined as: 4′,5,7-trihydroxyisoflavone(),4-(2,4-dihydroxybenzamido) benzoic acid (2),cyclo-(Gly-Ala)(3),Cyclo(Pro-Gly) (4),(cyclo)-(Leu-Tyr)(5),cyclo-(Val-Leu)(6),cyclo-(Val-Ile)(7),cyclo-(Phe-Gly)(8),respectively.
CONCLUSIONS:
The antitumor activities of these compounds were assayed by the SRB method against K562 cell line.Compounds 4, 5 and 7 showed cytotoxicity at the concentration of 10 μmol/L.
Journal of Mass Spectrometry Volume 33, Issue 1, pages 25–34, January 1998
Energetics of the cyclo(Pro–Gly) cation fragmentation: a mass spectrometric study and theoretical calculations[Reference:
WebLink]
METHODS AND RESULTS:
The energetics of HNCO elimination from the Cyclo(Pro-Gly) radical cation was studied by electron ionization tandem mass spectrometry, photoionization mass spectrometry and RRKM/QET modeling. The heat of formation and vibrational frequencies of the reactant were calculated by density functional theory (DFT). Homodesmotic reactions involving Cyclo(Pro-Gly) were used to derive its heat of formation at 0 K as -72.0±3.0 kcal mol-1 (1 kcal=4.184 kJ). Vibrational frequencies were calculated at the B3LYP/6–31G(d) level. The critical energy for HNCO loss was determined from the experiment, by modeling, to be 1.2±0.1 eV. The heat of formation of the Cyclo(Pro-Gly) radical cation and of the product ion C6H9NO+• at 0 K were determined to be 130.9±3.0 and ⩽182.8±5.0 kcal mol-1, respectively.
CONCLUSIONS:
The salient features of the potential energy profile along the reaction coordinate were probed by DFT calculations.