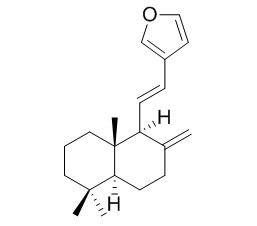
Coronarin E
Coronarin E exhibits weak antimicrobial activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Chem.2019, 279:80-87
BMC Complement Med Ther. 2020, 20(1):91.
Food Chem.2020, 313:126079
BMB Rep.2020, 53(4):218-222.
Front Pharmacol.2021, 12:615157.
Evid Based Complement Alternat Med.2022, 2022:3483511
Molecules.2024, 29(23):5792.
Korean J. Food Preserv.2023, 30(4):663-668.
BMC Microbiol.2019, 19(1):78
Sci Rep.2025, 15(1):29590.
Related and Featured Products
Asian J. Pharm. Clin. Res., 2015, 8(5):221-6.
Chemical composition and antimicrobial activity of diterpene and essential oils of hedychium roxburghii blume rhizome[Reference:
WebLink]
The objective of the present study was to isolate and determine diterpene compound and essential oils from Hedychium roxburghii Blume rhizome and investigated those antimicrobial activities.
METHODS AND RESULTS:
The essential oils were obtained by steam distillation method, the residual was then extracted by reflux with ethanol. The content of essential oils was analyzed by gas chromatography-mass spectrometry (GC/MS) method. Ethanolic residual-distillation extract was concentrated then used to isolate compound 1 by vacuum liquid chromatography and centrifugal chromatography. It was characterized by infrared spectrophotometry, 1H-nuclear magnetic resonance (NMR), 13C-NMR, heteronuclear single quantum coherence-NMR, heteronuclear multiple bond correlation-NMR and carbon coupling 13C-NMR. The antimicrobial activity of essential oils, ethanolic residual-distillation extract and compound 1 were carried out by microdilution method. The oils exhibited antimicrobial activity against Bacillus subtilis American Type Culture Collection (ATCC) 6633 (minimum inhibitory concentration [MIC] 1750 μg/ml), Staphylococcus aureus ATCC 6538 (MIC 1750 μg/ml), Escherichia coli ATCC 8939 (MIC 3500 μg/ml), Pseudomonas aeruginosa ATCC 9027 (>3500 μg/ml) and Candida albicans ATCC 10231 (MIC 875 μg/ml). A phytochemical study of the rhizome essential oils of H. roxburghii Blume were performed by GC/MS and the result showed that fenchyl acetate (45.85%) was the main component of the oils. Compound 1 was identified as diterpene compound, Coronarin E. Coronarin E have not exhibited MIC at 512 μg/ml, however, it showed inhibition profile against all of tested microbes.
CONCLUSIONS:
The essential oils and ethanolic residual-distillation extract of H. roxburghii Blume rhizome exhibited weak antimicrobial profile. Compound 1 was identified as diterpene compound, (Coronarin E), it was exhibited weak antimicrobial activity, but showed inhibition profile against all of the tested microbes.
Chem Pharm Bull (Tokyo). 2008 Mar;56(3):398-403.
Concise syntheses of coronarin A, coronarin E, austrochaparol and pacovatinin A.[Pubmed:
18310958]
Total syntheses of (+)-coronarin A (1), (+)-Coronarin E (2), (+)-austrochaparol (3) and (+)-pacovatinin A (4) were achieved from the synthetic (+)-albicanyl acetate (6).
METHODS AND RESULTS:
Dess-Martin oxidation of (+)-albicanol (5) derived from the chemoenzymatic product (6) gave an aldehyde (7), which was subjected to Julia one-pot olefination using beta-furylmethyl-heteroaromatic sulfones (8 or 9 ) gave (+)-trans Coronarin E (2) and (+)-cis Coronarin E (12) with high cis-selectivity. The synthesis of (+)-coronarin A (1) from (+)-trans Coronarin E (2) was achiev-ed, while (+)-cis Coronarin E (12) was converted to the natural products (+)-(5S,9S,10S)-15,16-epoxy-8(17),13(16),14-labdatriene (13) and (+)-austrochaparol (3).
CONCLUSIONS:
By the asymmetric synthesis of (+)-3, the absolute structure of (+)-3 was determined to be 5S, 7R, 9R, 10S configurations. Homologation of (+)-albicanol (5) followed by allylic oxidation gave (7 alpha)-hydroxy nitrile (17), which was finally converted to the natural (+)-pacovatinin A (4) in 8 steps from (+)-albicanol (5).