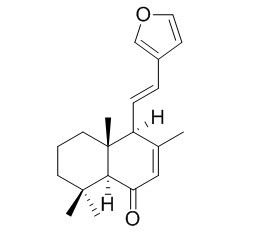
Hedychenone
Hedychenone has anti-inflammatory activity, it also shows potent in vitro cytotoxic activity against cancerous cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Vietnam J. Chem.2023, 61(3),308-317
Pharmacognosy Journal.2022, 14,4,327-337.
Biosci Rep.2018, 38(4)
Curr Res Food Sci.2024, 9:100827.
Applied Biological Chemistry2022, 65(85).
Pharmaceuticals (Basel).2024 Feb 24;17(3):292.
Industrial Crops and Products2017, 95:286-295
Food Chem X.2024, 24:101794.
Biol Pharm Bull.2023, 46(2):245-256.
Korean J. Medicinal Crop Sci.2021, 29(6):425-433
Related and Featured Products
Bioorg Med Chem Lett. 2010 Apr 15;20(8):2525-8.
Synthesis, cytotoxic activity and structure-activity relationships of hedychenone analogues.[Pubmed:
20303755]
Hedychenone, a plant-derived labdane diterpenoid, showed potent in vitro cytotoxic activity against cancerous cells.
METHODS AND RESULTS:
In the present study, a series of analogues have been synthesized by modification of the furanoid ring, double bond and the vinylic methyl functionality of this natural product lead and evaluated for their cytotoxic activities against human cancer cell lines. The structures of the target compounds were established by IR, (1)H NMR and mass spectral analysis. Majority of the analogues displayed potent activity than the parent compound, Hedychenone.
CONCLUSIONS:
Preliminary structure-activity relationship studies indicated that furanoid ring has a greater impact on cytotoxicity than that of the decalone nucleus. However, dimerization through C-8 significantly enhanced the cytotoxic activity of the Hedychenone.
Journal of Planar Chromatography - Modern TLC, 2007 , 20 (1) :73-74.
A new, convenient method for quantitative analysis of hedychenone, an anti-inflammatory compound in the rhizomes of Hedychium spicatum (Buch-Hem)[Reference:
WebLink]
Plants of the Hedychium genus are perennial rhizomatus plants belonging to the Zingiberaceae family. Extracts of Zingiberacaea have long been used in traditional medicine.
METHODS AND RESULTS:
A simple, precise, and convenient HPTLC method has been established for analysis of Hedychenone, the major marker compound extracted from the rhizomes of Hedychium spicatum (Buch-Hem). Chromatography was performed on silica gel 60F 254 plates with ethyl acetate-hexane, 20 + 80 ( v/v ), as mobile phase. Detection and quantification were performed densitometrically at λ max = 254 nm with Hedychenone as external standard.
CONCLUSIONS:
The method is characterized by high sensitivity and linearity over a wide range of concentrations.