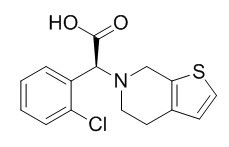
Clopidogrel Related Compound A
Clopidogrel Related Compound A is a impurity from Clopidogrel.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Naunyn Schmiedebergs Arch Pharmacol.2024, 03148-x.
J Cell Mol Med.2022, 26(23):5807-5819.
Eur Rev Med Pharmacol Sci.2020, 24(9):5127-5139.
Journal of Functional Foods2021, 84:104581
Biomol Ther (Seoul).2020, 28(6):542-548.
Plants (Basel).2021, 10(6):1119.
Evid Based Complement Alternat Med.2018, 2018:4259603
Applied Biological Chem. 2020, 26(63).
Fitoterapia.2022, 105141.
Environ Toxicol.2020, doi: 10.1002
Related and Featured Products
Der Pharma Chemica;2010,2(4):244.
Non chiral High Performance Liquid Chromatography method for monitoring unknown impurities generated during stability of Clopidogrel tablets[Reference:
WebLink]
The purpose of this study was to develop a High performance liquid chromatography (HPLC) method for separating the unknown impurities generated during the accelerated stability storage of Clopidogrel Bisulphate Tablets. Also, we used the newly developed method to identify the factors that contribute to the formation of these unknown impurities in the tablet formulation.
METHODS AND RESULTS:
Study was carried out by incubation of mixture of excipients and Clopidogrel API in 5:1 ratio at 80°C for 3days. The new HPLC method was developed by using Kromasil 100 C18 column and using gradient method with mobile phase of 0.1% Triflouroacetic acid in water in pump A and 0.1% Trifluoroacetic acid in Acetonitrile in pump B was suitable for separating the unknown impurities from the Clopidogrel Related Compound A.The method discussed in United States Pharmacopoeia (USP) is not suitable to separate these impurities. From the excipients compatibility data we hypothesized that these unknown impurities were generated due to the excipient Polyethylene Glycol that is present in the tablet both as a tablet lubricant as well as a part of the film coating system. Further these unknown impurities were characterized as Dihydro pyridinone Derivative, Decarbmethoxylated Clopidogrel.