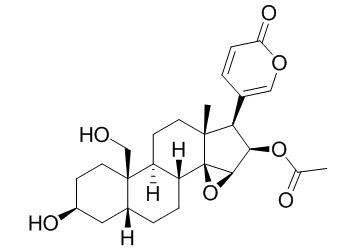
Cinobufaginol
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
SSRN2024, 4937625.
Food Res Int.2020, 128:108778
Current Enzyme Inhibition2023, 19(1):55-64(10)
Chem Biol Interact.2022, 368:110248.
Drug Des Devel Ther.2020, 14:5189-5204.
Revista Brasileira de Farmacognosia2024, 34:1091-1100.
BMC Complement Altern Med.2014, 14:242
Mal J Med Health Sci.2024, 20(SUPP5):151-156.
Biomolecules2021, 11(10),1513.
Kyung Hee University2024, rs-3888374
Related and Featured Products
Chemical Research in Chinese Universities, 2009, 25(6):801-806.
Simultaneous Determination of Bufadienolides and Qualitative Evaluation for Venenum Bufonis by High Performance Liquid Chromatography[Reference:
WebLink]
A high performance liquid chromatographic method was used for the simultaneous identification and qua-litative evaluation of 12 bufadienolides(resibufogenin, cinobufagin, Cinobufaginol, arenobufagin, bufalin, bufotalin, gamabufotalin, cinobufotalin, Ψ-bufaranogin, desacetylcinobufagin, telocinobufagin and resibufogenol) in Venenum Bufonis.
METHODS AND RESULTS:
The chromatographic separation was performed on a Dikma C 18 analytical column via gradient elution with an aqueous solution of acetonitrile and 0.3% acetic acid at a flow rate of 0.8 mL/min. The method was validated to be acceptable in consideration of linearity(r 2 > 0.9992) and recovery(ranged from 98.9% to 102.0%). The limits of de-tection of the bufadienolides were from 0.48 ng for bufalin to 6.00 ng for cinobufotalin. The intra-day and inter-day precisions of the method were evaluated and were less than 3.0%. The method was successfully used to analyze 19 batches of Venenum Bufonis, and the similarity values between batches were calculated by Similarity Evaluation System for Chromatographic Fingerprint of TCM(Version 2004A, Chinese Pharmacopoeia Committee, Beijing).
CONCLUSIONS:
The results show that the contents of bufadienolides in the medicine and the similarity values based on these bufadieno-lides varied significantly from batch to batch. This proposed method could be utilized to qualify and control Venenum Bufonis to ensure its safety and efficacy in application.