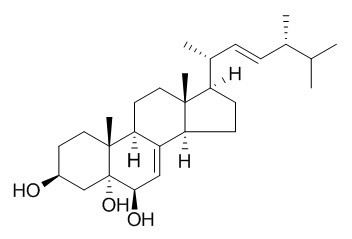
Cerevisterol
Cerevisterol is a cytotoxic steroid, can inhibit the activity of DNA polymerase alpha. It can stimulate NGF-mediated neurite outgrowth on PC12 cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Life Sci.2018, 209:498-506
J of the Society of Cosmetic Scientists of Korea2018, 44(4):407-417
Microbiol. Biotechnol. Lett.2022, 50(2): 193-201.
Adv Healthc Mater.2024, 13(13):e2303276.
J Funct Foods2019, 54:449-456
Pharmaceuticals (Basel). 2021, 14(10):986.
Natural Product Communications2020, doi: 10.1177.
Food Funct.2022, 13(13):6923-6933.
J.Soc.Cosmet.Sci.Korea2024, 50(3): 261-270
Cell Rep.2020, 32(11):108158.
Related and Featured Products
Nat Prod Res. 2012 Nov;26(21):2013-9.
An antimicrobial diketopiperazine alkaloid and co-metabolites from an endophytic strain of Gliocladium isolated from Strychnos cf. toxifera.[Pubmed:
22117164]
METHODS AND RESULTS:
From an endophytic strain of Gliocladium sp. isolated from the Amazonian plant Strychnos cf. toxifera, we obtained the diketopiperazine alkaloid cyclo-(glycyl-L-tyrosyl)-4,4-dimethylallyl ether (1), the steroids ergosterol (2), ergosterol peroxide (3), Cerevisterol (4) and the citric acid (5). The AcOEt extract of the fermented broth by Gliocladium sp. showed potent activity against the cancer cell lines MDA-MB435 (human breast cancer cells), HCT-8 (human colorectal cancer cells) and SF-295 (human glioblastoma cancer cells).
CONCLUSIONS:
Compound 1 exhibited a strong antimicrobial activity against Micrococcus luteus at a concentration of 43.4 µM.
Bioorg Med Chem. 1999 Sep;7(9):2047-52.
Lucidenic acid O and lactone, new terpene inhibitors of eukaryotic DNA polymerases from a basidiomycete, Ganoderma lucidum.[Pubmed:
10530954]
METHODS AND RESULTS:
Terpenoids, 1, 2 and 3, which selectively inhibit eukaryotic DNA polymerase activities, were isolated from the fruiting body of a basidiomycete, Ganoderma lucidum, and their structures were determined by spectroscopic analyses. New terpenes, lucidenic acid O (1) and lucidenic lactone (2), prevented not only the activities of calf DNA polymerase alpha and rat DNA polymerase beta, but also these of human immunodeficiency virus type 1 reverse transcriptase.
CONCLUSIONS:
Cerevisterol (3), which was reported to be a cytotoxic steroid, inhibited only the activity of DNA polymerase alpha. Although these compounds did not influence the activities of prokaryotic DNA polymerases and other DNA metabolic enzymes such as T7 RNA polymerase and deoxyribonuclease I.
Zhong Yao Cai. 2014 Feb;37(2):266-9.
Chemical constituents from Dichotella gemmacea.[Pubmed:
25095349]
To study the chemical constituents from Dichotella gemmacea.
METHODS AND RESULTS:
Chemical constituents were isolated by silica gel and Sephadex LH-20 column chromatography. The structures of the isolated compounds were elucidated through spectroscopic analysis.
Eight compounds were identified as fragilide J(I), junceelldide D(II), junceellin A (IlI), juncin P(IV), dichotellides A (V), Cerevisterol (VI), 1,2-diphenyldiselane (VII) and 5H-pyrido[4,3-b] indole (VIII).
CONCLUSIONS:
Compounds VI, VII and VIII are isolated from Dichotella gemmacea for the first time.
Bioorg. Med. Chem. Lett., 2015, 25(22):5078-82.
Chemical constituents from Hericium erinaceus and their ability to stimulate NGF-mediated neurite outgrowth on PC12 cells.[Pubmed:
26481911]
METHODS AND RESULTS:
One new meroterpenoid, named hericenone K (11), along with 10 known compounds (1-10), ergosterol peroxide (1), Cerevisterol (2), 3β,5α,9α-trihydroxy-ergosta-7,22-dien-6-one (3), inoterpene A (4), astradoric acid C (5), betulin (6), oleanolic acid (7), ursolic acid (8), hemisceramide (9), and 3,4-dihydro-5-methoxy-2-methyl-2-(4'-methyl-2'-oxo-3'-pentenyl)-9(7H)-oxo-2H-furo[3,4-h]benzopyran (10), was isolated from the fruiting bodies of the mushroom Hericium erinaceus. Their structures were characterized on the basis of spectroscopic methods, as well as through comparison with previously reported data.
CONCLUSIONS:
Compounds 3-6, 8, and 9 were isolated from Hericium species for the first time. Compounds 10 and 11 was suggested to be racemic by the CD spectrum data and specific rotations, which ware resolved by chiral HPLC into respective enantiomers. Compounds 1-3, (±)-10, (-)-10 and (+)-10 in the presence of NGF (20 ng/mL) exerted a significant increase in neurite-bearing cells.