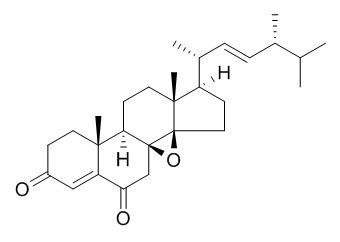
8,14-Epoxyergosta-4,22-diene-3,6-dione
(22E,24R)-8,14-epoxyergosta-4,22-diene-3,6-dione has antitumor activity, it may be disarming oncogenic pathways via direct modulation of the epigenetic machinery.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Res Int.2017, 96:40-45
Agriculture2022, 12(12), 2173.
J Appl Biol Chem.2024, 67:33,238-244
American Association for Anatomy2020, doi: 10.1002.
Pharmacognosy Journal2019, 11,6:1235-1241
Processes 2021, 9(5),894.
Acta Edulis Fungi2020, 27(02):63-76.
Int J Mol Sci.2018, 19(2)
Compounds.2023, 3(1), 169-179.
Curr Res Virol Sci.2022, 3:100019.
Related and Featured Products
Ergosta-4,6,8(14),22-tetraen-3-one
Catalog No: CFN99889
CAS No: 19254-69-4
Price: Inquiry(manager@chemfaces.com)
Cerevisterol
Catalog No: CFN98825
CAS No: 516-37-0
Price: Inquiry(manager@chemfaces.com)
6-O-Methylcerevisterol
Catalog No: CFN99372
CAS No: 126060-09-1
Price: Inquiry(manager@chemfaces.com)
3,5-Dihydroxyergosta-7,22-dien-6-one
Catalog No: CFN99614
CAS No: 14858-07-2
Price: Inquiry(manager@chemfaces.com)
3,5,9-Trihydroxyergosta-7,22-dien-6-one
Catalog No: CFN97449
CAS No: 88191-14-4
Price: Inquiry(manager@chemfaces.com)
Makisterone A 20,22-monoacetonide
Catalog No: CFN96788
CAS No: 245323-24-4
Price: Inquiry(manager@chemfaces.com)
8,14-Epoxyergosta-4,22-diene-3,6-dione
Catalog No: CFN97834
CAS No: 1265908-20-0
Price: Inquiry(manager@chemfaces.com)
3,5-Cycloergosta-6,8(14),22-triene
Catalog No: CFN98257
CAS No: 24352-51-0
Price: Inquiry(manager@chemfaces.com)
5,8-Epidioxyergosta-6,9(11),22-trien-3-ol
Catalog No: CFN93118
CAS No: 86363-50-0
Price: Inquiry(manager@chemfaces.com)
Ergosterol peroxide
Catalog No: CFN98035
CAS No: 2061-64-5
Price: $288/10mg
Chemistry & Biodiversity, 2010, 7(12):2941-2950.
Chemical Constituents of Papulaspora immersa, an Endophyte from Smallanthus sonchifolius (Asteraceae), and Their Cytotoxic Activity.[Reference:
WebLink]
Papulaspora immersa H. H. Hotson was isolated from roots and leaves of Smallanthus sonchifolius (Poepp. and Endl.) H. Rob. (Asteraceae), traditionally known as Yacon.
METHODS AND RESULTS:
The fungus was cultured in rice, and, from the AcOEt fraction, 14 compounds were isolated. Among them, (22E,24R)-8,14-Epoxyergosta-4,22-diene-3,6-dione (4), 2,3-epoxy-1,2,3,4-tetrahydronaphthalene-c-1,c-4,8-triol (10), and the chromone papulasporin (13) were new secondary metabolites. The spectral data of the known natural products were compared with the literature data, and their structures were established as the (24R)-stigmast-4-en-3-one (1), 24-methylenecycloartan-3β-ol (2), (22E,24R)-ergosta-4,6,8(14),22-tetraen-3-one (3), (-)-(3R,4R)-4-hydroxymellein (5), (-)-(3R)-5-hydroxymellein (6), 6,8-dihydroxy-3-methylisocoumarin (7), (-)-(4S)-4,8-dihydroxy-α-tetralone (8), naphthalene-1,8-diol (9), 6,7,8-trihydroxy-3-methylisocoumarin (11), 7-hydroxy-2,5-dimethylchromone (12), and tyrosol (14).
CONCLUSIONS:
Compound 4 showed the highest cytotoxic activity against the human tumor cell lines MDA-MB435 (melanoma), HCT-8 (colon), SF295 (glioblastoma), and HL-60 (promyelocytic leukemia), with IC₅₀ values of 3.3, 14.7, 5.0 and 1.6 μM, respectively. Strong synergistic effects were also observed with compound 5 and some of the isolated steroidal compounds.
Planta Medica, 2014, 80(06):473-481.
The anti-promyelocytic leukemia mode of action of two endophytic secondary metabolites unveiled by a proteomic approach.[Reference:
WebLink]
METHODS AND RESULTS:
As a result of a program to find antitumor compounds of endophytes from medicinal Asteraceae, the steroid (22E,24R)-8,14-Epoxyergosta-4,22-diene-3,6-dione (a) and the diterpene aphidicolin (b) were isolated from the filamentous fungi Papulaspora immersa and Nigrospora sphaerica, respectively, and exhibited strong cytotoxicity against HL-60 cells. A proteomic approach was used in an attempt to identify the drugs' molecular targets and their respective antiproliferative mode of action. Results suggested that the (a) growth inhibition effect occurs by G2/M cell cycle arrest via reduction of tubulin alpha and beta isomers and 14-3-3 protein gamma expression, followed by a decrease of apoptotic and inflammatory proteins, culminating in mitochondrial oxidative damage that triggered autophagy-associated cell death. Moreover, the decrease observed in the expression levels of several types of histones indicated that (a) might be disarming oncogenic pathways via direct modulation of the epigenetic machinery.
CONCLUSIONS:
Effects on cell cycle progression and induction of apoptosis caused by (b) were confirmed. In addition, protein expression profiles also revealed that aphidicolin is able to influence microtubule dynamics, modulate proteasome activator complex expression, and control the inflammatory cascade through overexpression of thymosin beta 4, RhoGDI2, and 14-3-3 proteins. Transmission electron micrographs of (b)-treated cells unveiled dose-dependent morphological characteristics of autophagy- or oncosis-like cell death.