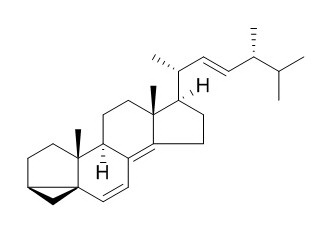
3,5-Cycloergosta-6,8(14),22-triene
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
South African J of Plant&Soil2018, 29-32
National University of Pharmacy2022, 1:73-76
Foods.2023, 12(6):1227.
J Biomol Struct Dyn.2023, 1-21.
Mol Divers.2022, s11030-022-10586-3.
Phytomedicine.2022, 100:154058.
Evid Based Complement Alternat Med.2020, 2020:1970349.
Front Pharmacol.2022, 13:906763.
Journal of Life Science2018, 917-922
J Agric Food Chem.2021, 69(46):14037-14047.
Related and Featured Products
Journal of the American Chemical Society, 1952, 74(21):469-74.
An i-Steroid Hydrocarbon from Ergosterol1.[Reference:
WebLink]
METHODS AND RESULTS:
Reinvestigation of a hydrocarbon resulting from dehydration of ergosterol with either phosphorus oxychloride or p-toluenesulfonyl chloride in pyridine and heretofore regarded as an ergostatetraene has shown that the substance is 3,5-cyclo-Δ6, 8(14), 22-ergostatriene (3,5-Cycloergosta-6,8(14),22-triene, V), the first known i-steroid in the ergosterol series. Reaction of the hydrocarbon with hydrogen chloride and its acid-catalyzed hydration result in opening of the cyclopropane ring with formation of mixtures of ergosteryl chlorides or of ergosterol isomers; ergosterol-B1 of reasonable purity has been isolated from the product of hydration. A hydrocarbon, first described by Windaus, resulting from acid-catalyzed dehydration of 3α- and 3β-hydroxy-Δ4,7,22-ergostatriene was investigated to see if it corresponded to a highly dextrorotatory, alumina-isomerized impurity accompanying the i-steroid V.
CONCLUSIONS:
The results indicate that the Windaus hydrocarbon is Δ4,6,8(14),22-ergostatetraene (XIV). Formulas attributed earlier to hydrocarbons resulting from dehydration of epialloergosterol (NaOAc-Ac2O, see III) and of lumisterol (C10-epimer of III) are consistent with present evidence. "Ergosterol-E" is shown to consist largely of a mixture of dihydroergosterols.