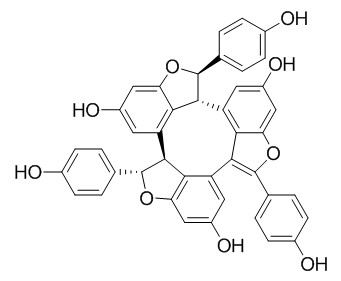
Caraphenol A
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Chengdu University of Traditional Chinese Medicine2024, 4802935.
Antioxidants (Basel).2021, 10(10):1620.
Primary and Industrial.2018, 52(11)
Nutrients.2021, 13(1):254.
Sichuan Agricultural University2023, 4630743.
Front Cell Dev Biol.2021, 9:764263.
Med Sci Monit.2019, 25:9499-9508
Bioorg Chem.2024, 145:107182.
Research Square2024, 4805471.
Molecules.2021, 26(6):1738.
Related and Featured Products
Angew Chem Int Ed Engl. 2014 Mar 24;53(13):3409-13.
9-Membered carbocycle formation: development of distinct Friedel-Crafts cyclizations and application to a scalable total synthesis of (±)-caraphenol A.[Pubmed:
24677499]
METHODS AND RESULTS:
Explorations into a series of different approaches for 9-membered carbocycle formation have afforded the first reported example of a 9-exo-dig ring closure via a Au(III)-promoted reaction between an alkyne and an aryl ring as well as several additional, unique Friedel-Crafts-type cyclizations. Analyses of the factors leading to the success of these transformations are provided, with the application of one of the developed 9-membered ring closures affording an efficient and scalable synthesis of the bioactive resveratrol trimer Caraphenol A.
CONCLUSIONS:
That synthesis proceeded with an average yield of 89% per step (7.8% overall yield) and has provided access to more than 600 mg of the target molecule.