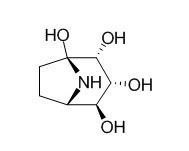
Calystegine B3
Calystegine B3 is a highly specific inhibitor for Man2C1 among various α-mannosidases prepared from rat liver, it could thus serve as a potent tool for the development of a highly specific in vivo inhibitor for Man2C1.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Nat Med.2017, 71(2):380-388
Chin. Med.J.Res. Prac.2017, 31(4)
J Nat Prod.2017, 80(4):854-863
Preprints2017, 2017120176
Biomedicines.2022, 10(5):1170
Separations2023, 10(4), 231.
J Pharm Anal.2016, 6(6):363-373
Front Chem.2023, 11:1245071.
Oncol Lett.2020, 20(4):122.
Molecules2022, 27(9):2992.
Related and Featured Products
J Biochem. 2011 Apr;149(4):415-22.
Calystegine B3 as a specific inhibitor for cytoplasmic alpha-mannosidase, Man2C1.[Pubmed:
21217149]
Development of a specific inhibitor for Man2C1 is critical to understanding its biological significance.
METHODS AND RESULTS:
In this study, we identified a plant-derived alkaloid, Calystegine B3, as a potent specific inhibitor for Man2C1 activity. Biochemical enzyme assay revealed that Calystegine B3 was a highly specific inhibitor for Man2C1 among various α-mannosidases prepared from rat liver. Consistent with this in vitro result, an in vivo experiment also showed that treatment of mammalian-derived cultured cells with this compound resulted in drastic change in both structure and quantity of free oligosaccharides in the cytosol, whereas no apparent change was seen in cell-surface oligosaccharides.
CONCLUSIONS:
Calystegine B3 could thus serve as a potent tool for the development of a highly specific in vivo inhibitor for Man2C1.
Bioorg Med Chem. 2014 Apr 15;22(8):2435-41.
Docking and SAR studies of calystegines: binding orientation and influence on pharmacological chaperone effects for Gaucher's disease.[Pubmed:
24657053]
We report on the identification of the required configuration and binding orientation of nor-tropane alkaloid calystegines against β-glucocerebrosidase.
METHODS AND RESULTS:
Calystegine B2 is a potent competitive inhibitor of human lysosomal β-glucocerebrosidase with Ki value of 3.3 μM. A molecular docking study revealed that calystegine B2 had a favorable van der Waals interactions (Phe128, Trp179, and Phe246) and the hydrogen bonding (Glu235, Glu340, Asp127, Trp179, Asn234, Trp381 and Asn396) was similar to that of isofagomine. All calystegine isomers bound into the same active site as calystegine B2 and the essential hydrogen bonds formed to Asp127, Glu235 and Glu340 were maintained. However, their binding orientations were obviously different. Calystegine A3 bound to β-glucocerebrosidase with the same orientations as calystegine B2 (Type 1), while Calystegine B3 and B4 had different binding orientations (Type 2). It is noteworthy that Type 1 orientated calystegines B2 and A3 effectively stabilized β-glucocerebrosidase, and consequently increased intracellular β-glucocerebrosidase activities in N370S fibroblasts, while Type 2 orientated calystegines B3 and B4 could not keep the enzyme activity.
CONCLUSIONS:
These results clearly indicate that the binding orientations of calystegines are changed by the configuration of the hydroxyl groups on the nor-tropane ring and the suitable binding orientation is a requirement for achieving a strong affinity to β-glucocerebrosidase.