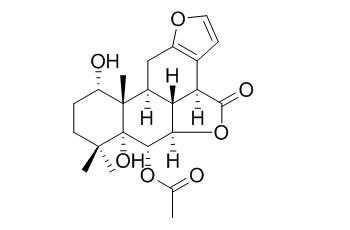
Bonducellpin D
Bonducellpin D may show inhibitory activities on the Para3 virus. It also exhibits moderate activity against four tested human cancer cell lines, HepG-2, K562, HeLa, and Du145.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J. Soc. Cosmet. Sci. Korea2016, 163-171
J Korean Med Ophthalmol Otolaryngol Dermatol2023, 36(1):21-39.
International Food Research Journal2018, 25(6):2560-2571
Asian J Beauty Cosmetol2022, 20(2):183-191
Int J Mol Sci.2022, 23(23):14826.
Evid Based Complement Alternat Med.2021, 8855980.
Life Sci.2023, 317:121458.
Phytochem Anal.2024, pca.3319.
J Nat Prod.2023, 86(2):264-275.
Cell Rep.2020, 32(11):108158.
Related and Featured Products
J Nat Prod. 2013 Dec 27;76(12):2210-8.
Caesalminaxins A-L, cassane diterpenoids from the seeds of Caesalpinia minax.[Pubmed:
24303808 ]
Fourteen new cassane diterpenoids, caesalminaxins A-L (1-14), and three known compounds were isolated from the seeds of Caesalpinia minax.
METHODS AND RESULTS:
Among the new diterpenoids, compounds 3 and 4 possess a rare spiro C/D ring system. The C-16 epimeric mixture 1/2 has an unprecedented carbon skeleton, presumably derived from 3 by cleavage of the C-13-C-14 bond. Compound 5 is the first example of a cassane diterpenoid with a spiro A/B ring system. The structures of the compounds were elucidated on the basis of 1D and 2D NMR analysis, and the absolute configurations of 3, 4, 9, and 11 were determined by single-crystal X-ray crystallography. Biosynthesis pathways for 1/2, 3, and 5 are postulated.
CONCLUSIONS:
Compounds 4, 8, and the known Bonducellpin D exhibited moderate activity against four tested human cancer cell lines, HepG-2, K562, HeLa, and Du145.
Bioorg Med Chem. 2002 Jul;10(7):2161-70.
Molecular structures and antiviral activities of naturally occurring and modified cassane furanoditerpenoids and friedelane triterpenoids from Caesalpinia minax.[Pubmed:
11983512]
METHODS AND RESULTS:
Further investigation of the active components of the chloroform fraction of the seeds of Caesalpinia minax led to the isolation of a new cassane furanoditerpenoid, caesalmin H (1), together with two known furanoditerpenoid lactones, caesalmin B (2) and Bonducellpin D (3). Reduction of the naturally abundant caesalmin D (9), E (10) and F (11) resulted in three new furanoditerpenoid derivatives 4-6. Phytochemical study of the stem of the same plant and subsequent reduction afforded two friedelane triterpenoids (7-8), which were identified by spectroscopic methods. Compounds 1-2 and 4-8 were corroborated by single crystal X-ray analysis.
CONCLUSIONS:
The factors governing the reduction of cassane furanoditerpenoids and friedelane triterpenoids were investigated by correlating the crystallographic results with density functional theory. The inhibitory activities of 2-8 on the Para3 virus were evaluated by cytopathogenic effects (CPE) reduction assay.