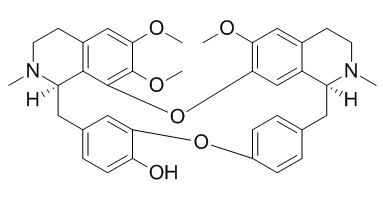
Berbamine
Berbamine is a novel inhibitor of bcr/abl fusion gene with potent anti-leukemia activity and also an inhibitor of NF-κB. Berbamine may be the first ATP-competitive inhibitor of CaMKII γ, could be as a new type of molecular targeted agent through inhibition of the CaMKII γ activity for treatment of leukemia.Berbamine confers cardioprotection against I/R injury by attenuating [Ca(2+)inf(i) overloading and preventing calpain activation through the activation of the PI3K-Akt-GSK3β pathway and, subsequently, opening of the mitoK(ATP) channel.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Korean Herb. Med. Inf.2021, 9(2):231-239.
Food Chem.2020, 327:126992.
Indian J Pharm Sci.2024, 86(2):736-741.
Pharmacognosy Journal.2020, 12(2), p232-235.
Chem Res Toxicol. 2022, acs.chemrestox.2c00049.
Plant Physiol.2023, 193(3):1758-1771.
J Ethnopharmacol.2025, 350:120002.
Cosmetics2021, 8(3),91.
Antioxidants (Basel).2020, 9(2): E119
J Food Sci.2024, 3841.17112.
Related and Featured Products
Anat Rec (Hoboken). 2014 May;297(5):802-9.
Berbamine enhances the antineoplastic activity of gemcitabine in pancreatic cancer cells by activating transforming growth factor-β/Smad signaling.[Pubmed:
24619961]
Drug-resistance to gemcitabine chemotherapy in pancreatic cancer is still an unsolved problem. Combinations of other chemotherapy drugs with gemcitabine have been shown to increase the efficacy of gemcitabine-based treatment.
METHODS AND RESULTS:
In this study, the effect of Berbamine on the antitumor activity of gemcitabine was evaluated in human pancreatic cancer cell lines Bxpc-3 and Panc-1, and the underlying mechanisms were explored.
Our results demonstrated that Berbamine exhibited a time- and dose-dependent inhibitory effect in the pancreatic cancer cell lines. Berbamine enhanced gemcitabine-induced cell growth inhibition and apoptosis in these cells. Combined treatment of Berbamine and gemcitabine resulted in down-regulation of anti-apoptotic proteins (Bcl-2, Bcl-xL) and up-regulation of pro-apoptotic proteins (Bax, Bid). More importantly, Berbamine treatment in combination with gemcitabine activated the transforming growth factor-β/Smad (TGF-β/Smad) signaling pathway, as a result of a decrease in Smad7 and an increase in transforming growth factor-β receptor II (TβRII) expression. Changes in downstream targets of Smad7, such as up-regulation of p21 and down-regulation of c-Myc and Cyclin D1 were also observed. Therefore, Berbamine could enhance the antitumor activity of gemcitabine by inhibiting cell growth and inducing apoptosis, possibly through the regulation of the expression of apoptosis-related proteins and the activation of TGF-β/Smad signaling pathway.
CONCLUSIONS:
Our study indicates that Berbamine may be a promising candidate to be used in combination with gemcitabine for pancreatic cancer treatment.
Circ J. 2012;76(8):1993-2002. Epub 2012 May 15.
Berbamine protects the heart from ischemia/reperfusion injury by maintaining cytosolic Ca(2+) homeostasis and preventing calpain activation.[Pubmed:
22664727]
Berbamine, a natural compound from Barberry, was reported to protect myocardium from ischemia/reperfusion (I/R) injury, but the underlying mechanisms are largely unknown.
METHODS AND RESULTS:
Berbamine pretreatment from 10 to 100nmol/L concentration-dependently improved post-ischemic myocardial function. Similar protection was confirmed in isolated cardiomyocytes characterized by the attenuation of I/R-induced intracellular free Ca(2+) concentration ([Ca(2+)](i)) overloading and the depression of cell shortening and Ca(2+) transients, which were partially mimicked but not augmented by calpain inhibitor calpeptin and abolished by mitochondrial ATP-sensitive potassium (mitoK(ATP) channel inhibitor 5-hydroxydecanoate (5-HD) and phosphoinositide 3-kinase (PI3K) inhibitor wortmannin. Consistently, I/R-induced increase of calpain activity and decrease of sarcoplasmic reticulum Ca(2+) ATPase (SERCA2) activity; and protein expression of SERCA2a, desmin, calpastatin and Akt was significantly attenuated by Berbamine. In addition, I/R-decreased Akt protein was reversed by calpeptin. Moreover, Berbamine further increased I/R-enhanced phosphorylation of Akt and glycogen synthase kinase-3β (GSK3β). These protections were abolished by wortmannin. Furthermore, Berbamine significantly attenuated I/R-induced lactate dehydrogenase release, infarct size and contractile dysfunction, and such cardioprotective actions were abolished by wortmannin and 5-HD or mimicked by glycogen synthase kinase-3β (GSK3β) inhibitor SB216763 but without additive effect.
CONCLUSIONS:
These findings suggest that Berbamine confers cardioprotection against I/R injury by attenuating [Ca(2+)inf(i) overloading and preventing calpain activation through the activation of the PI3K-Akt-GSK3β pathway and, subsequently, opening of the mitoK(ATP) channel.
Mol Cancer Ther. 2013 Oct;12(10):2067-77.
Berbamine inhibits the growth of liver cancer cells and cancer-initiating cells by targeting Ca2⁺/calmodulin-dependent protein kinase II.[Pubmed:
23960096 ]
Liver cancer is the third leading cause of cancer deaths worldwide but no effective treatment toward liver cancer is available so far. Therefore, there is an unmet medical need to identify novel therapies to efficiently treat liver cancer and improve the prognosis of this disease.
METHODS AND RESULTS:
Here, we report that Berbamine and one of its derivatives, bbd24, potently suppressed liver cancer cell proliferation and induced cancer cell death by targeting Ca(2+)/calmodulin-dependent protein kinase II (CAMKII). Furthermore, Berbamine inhibited the in vivo tumorigenicity of liver cancer cells in NOD/SCID mice and downregulated the self-renewal abilities of liver cancer-initiating cells. Chemical inhibition or short hairpin RNA-mediated knockdown of CAMKII recapitulated the effects of Berbamine, whereas overexpression of CAMKII promoted cancer cell proliferation and increased the resistance of liver cancer cells to Berbamine treatments. Western blot analyses of human liver cancer specimens showed that CAMKII was hyperphosphorylated in liver tumors compared with the paired peritumor tissues, which supports a role of CAMKII in promoting human liver cancer progression and the potential clinical use of Berbamine for liver cancer therapies.
CONCLUSIONS:
Our data suggest that Berbamine and its derivatives are promising agents to suppress liver cancer growth by targeting CAMKII.
Blood. 2012 Dec 6;120(24):4829-39.
CaMKII γ, a critical regulator of CML stem/progenitor cells, is a target of the natural product berbamine.CaMKII γ, a critical regulator of CML stem/progenitor cells, is a target of the natural product berbamine.[Pubmed:
23074277]
Bcr-Abl tyrosine kinase inhibitors (TKIs) have been a remarkable success for the treatment of Ph(+) chronic myeloid leukemia (CML). However, a significant proportion of patients treated with TKIs develop resistance because of leukemia stem cells (LSCs) and T315I mutant Bcr-Abl.
METHODS AND RESULTS:
Here we describe the unknown activity of the natural product Berbamine that efficiently eradicates LSCs and T315I mutant Bcr-Abl clones. Unexpectedly, we identify CaMKII γ as a specific and critical target of Berbamine for its antileukemia activity. Berbamine specifically binds to the ATP-binding pocket of CaMKII γ, inhibits its phosphorylation and triggers apoptosis of leukemia cells. More importantly, CaMKII γ is highly activated in LSCs but not in normal hematopoietic stem cells and coactivates LSC-related β-catenin and Stat3 signaling networks. The identification of CaMKII γ as a specific target of Berbamine and as a critical molecular switch regulating multiple LSC-related signaling pathways can explain the unique antileukemia activity of Berbamine.
CONCLUSIONS:
These findings also suggest that Berbamine may be the first ATP-competitive inhibitor of CaMKII γ, and potentially, can serve as a new type of molecular targeted agent through inhibition of the CaMKII γ activity for treatment of leukemia.
Rapid Commun Mass Spectrom. 2014 Jan 15;28(1):143-7.
Electrospray ionization mass spectrometry probing of binding affinity of berbamine, a flexible cyclic alkaloid from traditional Chinese medicine, with G-quadruplex DNA.[Pubmed:
24285399]
Classic G-quadruplex binders typically have a large aromatic core and interact with G-quadruplexes through π-π stacking with the quartets. There are rather few reports on natural flexible cyclic molecule from traditional Chinese medicine which has high binding affinity with G-quadruplex.
METHODS AND RESULTS:
Electrospray ionization mass spectrometry (ESI-MS) experiments were performed to evaluate the binding affinities of a natural alkaloid, Berbamine, with seven G-quadruplexes. Furthermore, we utilized autodock4 analysis to uncover the binding mode of Berbamine with the G-quadruplex.
ESI-MS experiments showed that Berbamine has the best binding affinity toward the (GGA)8 G-quadruplex compared with the other six G-quadruplexes. Autodock4 analysis indicated that Berbamine interacted with the (GGA)8 G-quadruplex through hydrogen bonding and van der Waals forces with a binding site at the lateral groove formed by DG8-DA9-DA15-DG16.
CONCLUSIONS:
In this study, we discovered a novel G-quadruplex binder, Berbamine, which has high binding affinity toward the (GGA)8 G-quadruplex. This study provided important clues regarding the probing of small molecule from traditional Chinese medicine which can target the G-quadruplex with high affinity.