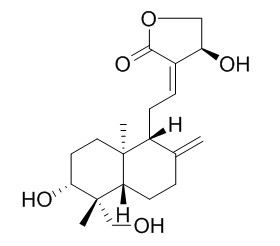
Andropanolide
Andropanolide is a natural product from Andrographis paniculata (Burm. f.) Nees.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Carbohydrate Polymer Technologies & App.2021, 2:100049.
Biomed Pharmacother.2024, 181:117647.
J Herbmed Pharmacol.2018, 7(4):280-286
Fitoterapia.2024, 175:105958.
J Food Sci.2022, 87(11):4905-4916.
United States Patent Application2020, 20200038363
Pharmacol Rep.2018, 70(6):1195-1201
J Agric Food Chem.2024, 72(42):23183-23195
Nutrients.2023, 15(13):2960.
Korean J. of Food Sci. and Tech2016, 172-177
Related and Featured Products
International Journal of Pharmacy & Pharmaceutical Sciences, 2014, 6(1):46–52.
MICROBIAL METABOLISM OF AN ANTI-HIV AND ANTI-MALARIAL NATURAL PRODUCT ANDROGRAPHOLIDE[Reference:
WebLink]
In the present study, we first time studied the microbial metabolism of andrographolide (1) with Cunninghamella elegans (TSY 0865) and Cephalosporium aphidicola (IMI-68689).
METHODS AND RESULTS:
Microbial cultures of the C. elegans and C. aphidicola were grown on Potato dextrose agar (PDA) at 25°C and stored at 4°C. Medium for C. aphidicola was prepared by mixing Glucose (50.0 g), KH2PO4 (1.0 g), MgSO4.7H2O (2.0 g), Glycin (2.0 g), KCl (1.0 g) and Gibberella trace element solution (2.0 mL) into distilled water (1 L) and maintained pH at 5.6. While C. elegans medium was prepared by adding Glucose (10.0 g), peptone (5.0 g), KH2PO4 .Two compounds were obtained as transformed products. Based on physical and spectroscopic data, these have been identified as Andropanolide (2) and 14-deoxy-11,12-didehydro andrographolide (3). Both compounds were previously obtained by the phytochemical investigation of A. paniculata and biotransformed product as well. (5.0 g), yeast extract (5.0 g), NaCl (5.0 g) and glycerol (10 mL) into distilled water (1 L) and maintained pH at 5.6.
CONCLUSIONS:
It could be concluded that C. elegans and C. aphidicola were able to produce oxidative derivatives of 1 in a regio- and stereoselective manner. Present investigation has been conducted for the first time with C. elegans and C. aphidicola. Incubation of 1 for 9 days with fungal strains yielded isomerized and oxidative products 2 and 3. Structures of all metabolites were elucidated by using spectroscopic techniques.
J Nat Prod. 2006 Mar;69(3):403-5.
Andropanolide and isoandrographolide, minor diterpenoids from Andrographis paniculata: structure and X-ray crystallographic analysis.[Pubmed:
16562845]
METHODS AND RESULTS:
Phytochemical investigation of the leaves of Andrographis paniculata has led to the isolation of a new labdane type diterpenoid, Andropanolide (1), along with seven known diterpenoids including isoandrographolide (2), previously reported as a rearrangement product of andrographolide. The structures and stereochemistry of compounds Andropanolide and 2 were established by X-ray crystallographic analysis.