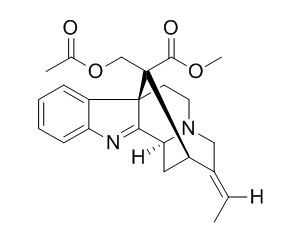
Akuammiline
Standard reference
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biomol Ther (Seoul).2023, 31(1):40-47.
J Biochem Mol Toxicol.2025, 39(8):e70416.
Pharmaceuticals (Basel). 2021, 14(10):986.
Food Bioscience2024, 58:103691.
Food and Fermentation Industries2018, 44(371)
Journal of Food Composition and Analysis2021, 100:103905.
Applied Biological Chemistry2023, 66:85.
Biomed Chromatogr.2016, 30(10):1573-81
Antioxidants (Basel).2023, 12(1):189.
LWT - Food Science and Technology2022, 164:113627
Related and Featured Products
J Nat Prod. 2007 Nov;70(11):1783-9.
Biologically active aspidofractinine, rhazinilam, akuammiline, and vincorine alkaloids from Kopsia.[Pubmed:
17939738]
METHODS AND RESULTS:
Eleven new indole alkaloids, in addition to the previously reported rhazinal (1), and 14 other known alkaloids, were obtained from the Malayan Kopsia singapurensis, viz., kopsiloscines A-F (2-7), 16-epikopsinine (8), kopsilongine- N-oxide (9), 16-epiAkuammiline (10), aspidophylline A (11), and vincophylline (12). The structures of these alkaloids were determined using NMR and MS analyses.
CONCLUSIONS:
Rhazinal (1), rhazinilam (17), and rhazinicine (18) showed appreciable cytotoxicity toward drug-sensitive as well as vincristine-resistant KB cells, while kopsiloscines A (2), B (3), and D (5) and aspidophylline A (11) were found to reverse drug-resistance in drug-resistant KB cells.
Angew Chem Int Ed Engl. 2015 Jan 7;54(2):400-12.
Cascade reactions: a driving force in akuammiline alkaloid total synthesis.[Pubmed:
25346244]
The Akuammiline alkaloids are a family of intricate natural products which have received considerable attention from scientists worldwide.
METHODS AND RESULTS:
Despite the fact that many members of this alkaloid class were discovered over 50 years ago, synthetic chemistry has been unable to address their architectures until recently. This minireview provides a brief overview of the rich history of the Akuammiline alkaloids, including their isolation, structural features, biological activity, and proposed biosyntheses. Furthermore, several recently completed total syntheses are discussed in detail. These examples not only serve to highlight modern achievements in alkaloid total synthesis, but also demonstrate how the molecular scaffolds of the
CONCLUSIONS:
Akuammilines have provided inspiration for the discovery and implementation of innovative cascade reactions for the rapid assembly of complex structures.