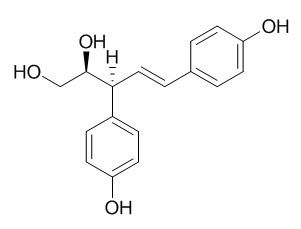
Agatharesinol
Agatharesinol is localized in the ray parenchyma cells in Sugi heartwood, and differences between the chemical structure of the contents of ray and axial parenchyma cells were also suggested.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biochem Biophys Res Commun.2021, 534:802-807.
Korean J Pain.2021, 34(4):405-416.
Iranian J. Pharm. Res.2021, 20(4):59-70
J Med Food.2019, 22(10):1067-1077
Molecules2022, 27(14):4601
Antioxidants (Basel).2020, 9(6):544.
J Pharm Anal.2016, 6(6):363-373
Immunopharmacol Immunotoxicol.2024, 46(4):496-508.
Evid Based Complement Alternat Med.2022, 9767292,2.
bioRxiv - Molecular Biology2023, 535548.
Related and Featured Products
Phytochemistry. 2002 Jul;60(5):461-6.
Immunohistochemical localization of agatharesinol, a heartwood norlignan, in Cryptomeria japonica.[Pubmed:
12052511]
METHODS AND RESULTS:
Localization of a heartwood norlignan, Agatharesinol, in Sugi (Japanese cedar, Cryptomeria japonica D. Don, Taxodiaceae) was investigated by immunohistochemistry. Immuno light microscopy showed that the contents of ray parenchyma cells were immunostained in heartwood but not in sapwood. The staining of the heartwood tissue was competitively inhibited by Agatharesinol but not by other Sugi heartwood extractives, and was, furthermore, markedly reduced by pre-extraction of the tissue with MeOH. These results indicated that the staining can be ascribed to the immunolabeling of Agatharesinol in situ. The accumulations over the inner surface of some tracheid cell walls adjacent to the ray parenchyma cells were also immunolabeled, while the contents in axial parenchyma cells were not.
CONCLUSIONS:
In conclusion, Agatharesinol was localized in the ray parenchyma cells in Sugi heartwood, and differences between the chemical structure of the contents of ray and axial parenchyma cells were also suggested.
J Plant Physiol. 2006 Mar;163(5):483-7.
Evidence for involvement of the phenylpropanoid pathway in the biosynthesis of the norlignan agatharesinol.[Pubmed:
16473652]
In order to study the biosynthesis of Agatharesinol, a norlignan, l-phenylalanine-[ring-2,3,4,5,6-2H] and trans-cinnamic acid-[ring-13C6] were administered to fresh sapwood sticks of Cryptomeria japonica (sugi, Japanese cedar), that is, the labeled precursors were allowed to be absorbed through the tangential section of the wood sticks.
METHODS AND RESULTS:
The wood sticks were then maintained in high humidity desiccators for approximately 20 d after which ethyl acetate (EtOAc) extracts of the wood sticks were analyzed by gas chromatography-mass spectrometry (GC-MS). Native Agatharesinol (trimethylsilylated) produces an m/z 369 ion and an m/z 484 ion that are characteristic of its structure. Agatharesinol formed in the sapwood sticks treated with the deuterium-labeled l-phenylalanine generated both of these ions together with m/z 373 and 377 ions (m/z 369+4 and +8, respectively), and also m/z 488 and 492 ions (m/z 484+4 and +8, respectively). Generation of m/z 373 and 488 ions is attributed to the substitution by deuterium of the four hydrogen atoms of either of the p-hydroxyphenyl rings of Agatharesinol, and that of m/z 377 and 492 ions is attributed to the substitution by deuterium of the eight hydrogen atoms of both p-hydroxyphenyl rings. In the administration of the 13C-labeled trans-cinnamic acid, m/z 375 and 381 ions (m/z 369+6 and +12, respectively), and also m/z 490 and 496 ions (m/z 484+6 and +12, respectively) were found, indicating that either aromatic ring or both aromatic rings of Agatharesinol were 13C-labeled.
CONCLUSIONS:
Consequently, assimilation of the labeled precursors into Agatharesinol was clearly detected, and an experimental procedure for studies on the biosynthesis was developed.