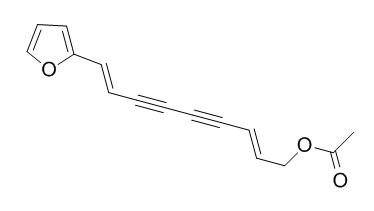
Acetylatractylodinol
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Agric Food Chem.2024,72(37):20396-20409.
Plant Physiol Biochem.2019, 144:355-364
Mol Biol Rep.2022, doi: 10.1007
Drug Des Devel Ther.2023, 17:2461-2479.
Nat Prod Sci.2018, 24(3):206
J Microbiol Biotechnol.2024, 35:e2408022.
Institute of Food Science & Technology2021, 18 December.
J of Health Science and Alternative Medicine2019, 1(1)
Front Cell Dev Biol.2021, 9:588093.
J Ethnopharmacol.2023, 321:117501.
Related and Featured Products
Planta Med. 2001 Jul;67(5):437-42.
Further phenols and polyacetylenes from the rhizomes of Atractylodes lancea and their anti-inflammatory activity.[Pubmed:
11488458 ]
METHODS AND RESULTS:
From the rhizomes of Atractylodes lancea, 2-[(2'E)-3',7'-dimethyl-2',6'-octadienyl]-4-methoxy-6-methylphenol (1) was isolated as a new natural product. The compound showed strong inhibitory effects on 5-lipoxygenase (5-LOX) and cyclooxygenase-1 (COX-1), but exhibited only weak antioxidative activities [IC50 = 0.1 microM (5-LOX), 2 microM (COX-1), 9 microM (PMN/FMLP), 28 microM (PMNIOZ)]. Moreover, five new acetylenes were isolated and elucidated as (3Z,5E,11E)-tridecatriene-7,9-diynyl-1-O-(E)-ferulate (2), erythro-(1,3Z,11E)-tridecatriene-7,9-diyne-5,6-diyl diacetate (3), (1Z)-atractylodin (4), (1Z)-atractylodinol (5), (1Z)-Acetylatractylodinol (6) plus the known (4E,6E,12E)-tetradecatriene-8,10-diyne-1,3-diyl diacetate (7).
CONCLUSIONS:
Among the acetylenes, only 2 showed strong inhibition of 5-LOX and COX-1 activity (IC50 (5-LOX) = 3 microM, IC50 (COX-1) = 1 microM).
In addition, the fatty acids linoleic acid, oleic acid and palmitic acid with previously established 5-LOX-/COX-1 inhibitory actions were identified as major constituents of the n-hexane extract and thus seem to contribute to the plant's in vitro activity.
Pharmaceutical and pharmacological letters,1998,8(2):69-71.
Antioxidant activity of constituents from Atractylodes lancea.[Reference:
WebLink]
Four polyacetylenes atractylodin, Acetylatractylodinol, 1-(2-furyl)-(7E)-nonene-3,5-diyne-1,2-diacetate, erythro-(1,5E, 11E)-tridecatriene-7,9-diyne-3,4-diacetate,as well as atractylochromene (I) and 2-[(2E)-3,7-dimethyl-2,6-octadienyl]-6-methyl-2,5-cyclohexadiene-1,4-dione (II) isolated from the rhizomes of Atractylodes lancea were evaluated for their antioxidant activity ex vivo.
METHODS AND RESULTS:
When measuring the inhibition of luminal enhanced chemiluminescence in stimulated human neutrophils, only the chromene I and the quinone II showed strong antioxidant activity. After stimulation with FMLP or opsonized zymosan (OZ) both compds. exhibited comparable inhibitory effects (IC50 I = 1.3 micro M (FMLP), 5.6 micro M (OZ); IC50 II = 1.1 micro M (FMLP), 5.4 micro M (OZ)). In contrast, I was considerably more active in a cell free in vitro assay with H2O2 and horseradish peroxidase (IC50 = 4.9 micro M I, 11 micro M II).