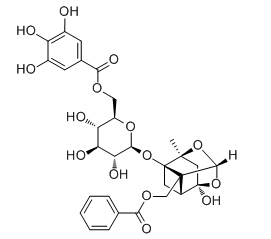
6'-O-Galloyl paeoniflorin
6'-O-Galloylpaeoniflorin (GPF) may be developed as a cytoprotector against ROS-mediated oxidative stress, GPF demonstrates a significant scavenging capacity against the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical, H2O2-generated intracellular reactive oxygen species (ROS), the superoxide anion radical (O2 -), and the hydroxyl radical (•OH); GPF also can safeguarde HaCaT keratinocytes against H2O2-provoked apoptotic cell death and attenuated oxidative macromolecular damage to DNA, lipids, and proteins; GPF exerts its cytoprotective actions in keratinocytes at least in part by decreasing the number of DNA strand breaks, the levels of 8-isoprostane (a stable end-product of lipid peroxidation), and the formation of carbonylated protein species. GPF shows strong androgen receptor (AR) binding activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2015, 16(8):18396-411
Chem Biol Interact.2016, 260:168-175
Cells.2023, 12(1):168.
Food Chem.2017, 228:301-314
Am J Chin Med.2023, 51(7):1675-1709.
Horticulture Research2022, uhac276.
Clin Exp Pharmacol Physiol.2015, 42(11):1189-97
Emirates Journal of Food and Agriculture.2022, 34(6): 528-536.
Pharmaceutics.2020, 12(9):845.
J Cell Mol Med.2023, 27(11):1592-1602.
Related and Featured Products
Biomol. Ther., 2013, 21(5):349-57.
6'-o-galloylpaeoniflorin protects human keratinocytes against oxidative stress-induced cell damage.[Pubmed:
24244822 ]
6'-O-Galloyl paeoniflorin (GPF) is a galloylated derivate of paeoniflorin and a key chemical constituent of the peony root, a perennial flowering plant that is widely used as an herbal medicine in East Asia.
METHODS AND RESULTS:
This study is the first investigation of the cytoprotective effects of GPF against hydrogen peroxide (H2O2)-induced cell injury and death in human HaCaT keratinocytes. GPF demonstrated a significant scavenging capacity against the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical, H2O2-generated intracellular reactive oxygen species (ROS), the superoxide anion radical (O2 (-)), and the hydroxyl radical (•OH). GPF also safeguarded HaCaT keratinocytes against H2O2-provoked apoptotic cell death and attenuated oxidative macromolecular damage to DNA, lipids, and proteins. The compound exerted its cytoprotective actions in keratinocytes at least in part by decreasing the number of DNA strand breaks, the levels of 8-isoprostane (a stable end-product of lipid peroxidation), and the formation of carbonylated protein species.
CONCLUSIONS:
Taken together, these results indicate that GPF may be developed as a cytoprotector against ROS-mediated oxidative stress.
Chem. Pharm. Bull., 2009, 57(9):971-4.
Androgen modulators from the roots of Paeonia lactiflora (paeoniae radix) grown and processed in nara prefecture, Japan.[Pubmed:
19721258]
The monoterpene glycoside, 3'-O-galloylpaeoniflorin (1), and four known compounds, 6'-O-galloylalbiflorin (2), pentagalloylglucose (3), 6'-O-benzoylpaeoniflorin (4) and 6'-O-Galloyl paeoniflorin (5), were isolated from the roots of Paeonia lactiflora that had been grown and processed in Nara prefecture, Japan, as androgen modulators.
METHODS AND RESULTS:
Their structures were elucidated based on spectroscopic analysis. Compounds 2 and 3 showed strong androgen receptor (AR) binding activity (IC(50) values 33.7 and 4.1 microg/ml, respectively), 1, 4 and 5 showed weak activity (20, 31 and 12% at 120 microg/ml, respectively). However, paeoniflorin (6) and albiflorin (7), the structures of which are related to 1, 2, 4 and 5, showed no activity.
CONCLUSIONS:
These results suggested that both the structure of albiflorin and the galloyl moiety are important for 2 to show strong AR binding activity. Furthermore, compounds 1-5 inhibited growth of an androgen-dependent LNCaP-FGC (prostate cancer cell line), and were indicated to be AR antagonists. Compounds 2 and 3 might be candidates as safe, natural anti-androgens.